This symposium took place on the 14th February 2020, as part of the 15th congress of the European Crohn’s and Colitis Organisation (ECCO) in Vienna, Austria
Chairperson: Fraser Cummings1
Speakers: Marc Ferrante,2 Gionata Fiorino3
1. University Hospital Southampton and University of Southampton, Southampton, UK
2. Katholieke Universiteit (KU) Leuven, Leuven, Belgium
3. Humanitas University and Humanitas Research Hospital, Milan, Italy
Disclosure: Dr Cummings has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Biogen, Celltrion, Falk, Ferring, Janssen, MSD, Napp Pharmaceuticals, Pfizer, Pharmacosmos, Samsung, Sandoz, and Takeda, and has received research funding from Amgen, AstraZeneca, Biogen, GSK, Hospira/Pfizer, and Janssen. Dr Ferrante has served as a consultant or speaker for AbbVie, Amgen, Biogen, Boehringer Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, Sandoz, Takeda, and Thermo Fisher, and has received research funding from Amgen, Biogen, Janssen, Pfizer, and Takeda. Dr Fiorino has served as a consultant and advisory board member for AbbVie, AlfaSigma, Amgen, Celltrion, Ferring, Janssen, MSD, Pfizer, Roche, Samsung, Sandoz, and Takeda.
Acknowledgements: Writing assistance was provided by Rebecca Bridgewater-Gill, Ascend, Spirit Medical Communications Group, Manchester, UK.
Support: The symposium and the publication of this article was funded by Amgen. This article was written by a medical writer and based only on the speaker presentations; the views and opinions expressed are not necessarily those of Amgen or the speakers. The speakers did not review this article.
Meeting Summary
Crohn’s disease (CD) and ulcerative colitis (UC) are progressive inflammatory diseases, and early intervention with biologics has been shown to slow disease progression and improve long-term outcomes. In CD, a growing body of evidence has demonstrated that introduction of anti-TNF therapies early in the disease course is associated with improved clinical outcomes when compared with later introduction of anti-TNF or use of conventional therapy. In UC, however, the data are very limited. Early mucosal healing in UC is associated with improved long-term outcomes, and earlier introduction of biologics over time has been paralleled by a decrease in colectomy rates. However, prospective, interventional studies assessing early intervention with anti-TNF in inflammatory bowel diseases (IBD) are lacking.
Several barriers exist that may limit the use of early intervention with anti-TNF in UC, including early diagnosis, the identification of patients who may benefit the most from early intervention, and misperceptions about UC management. The cost of anti-TNF may also limit their use early in the disease course, but the cost-effectiveness of biosimilars compared with originator anti-TNF could improve access.
Treatment targets are an important consideration in the management of early UC. Histological remission is a more stringent endpoint than the current goals of clinical and endoscopic remission, and is associated with lower rates of relapse and cancer. Although further validation of this treatment goal is required, treating to an appropriate target with the right drug early in the disease course could be critical to the management of UC.
Introduction
In an interactive symposium moderated by Dr Cummings, the question: “Is early intervention with anti-TNF the key to achieving long-lasting remission in patients with UC?” was debated by Dr Ferrante and Dr Fiorino.
CD and UC are progressive, inflammatory diseases, in which very early changes in the immune response occur even before tissue changes become apparent.1 In CD, the majority of patients present with predominantly inflammatory disease at diagnosis, and at 10 years after diagnosis, more than one-half of patients have stricturing or penetrating disease phenotypes, reaffirming the progressive nature of the disease.2
In the early stages of IBD, a ‘window of opportunity’ exists, during which there is the potential to change the disease course through pharmacological intervention.3 While there is more evidence for this in CD, an improved understanding of UC, indicating that it too has a progressive course, is leading experts to suggest that early intervention may also play a part in the management of this disease.4,5
Early Intervention with Anti-TNF in Inflammatory Bowel Disease: The Evidence
Much of the evidence for early intervention with anti-TNF in IBD comes from studies in patients with CD. In a post hoc subanalysis of patients with moderately-to-severely active CD treated with adalimumab in the Phase III ADHERE study (n=328), those with shorter disease duration (<2 years) had numerically higher rates of clinical remission (defined as Crohn’s Disease Activity Index [CDAI] <150) for up to 3 years compared with those with longer disease duration.6
Similar results were seen in the open-label ‘Step-Up/Top-Down’ study, in which patients with newly diagnosed CD who were naïve to immunomodulators or biologic therapy (N=133) were treated with either ‘top-down’ therapy (initial infliximab in combination with azathioprine) or ‘step-up’ therapy (corticosteroids with step-up to immunomodulator then infliximab as required).7 In this study, the rate of remission without corticosteroids or surgical resection was significantly higher in patients initially treated with infliximab and azathioprine (top-down therapy) than in those treated with step-up therapy at Weeks 26 (60.0% versus 35.9%; p=0.006) and 52 (61.5% versus 42.2%; p=0.028).
However, a retrospective review of long-term (8-year) outcomes of the Step-Up/Top-Down study (n=119) showed a more limited benefit of early intervention. Although the top-down strategy was numerically superior to the step-up strategy in terms of CD-related hospitalisation, new fistula formation, and CD-related surgery, statistical significance was only reached for the proportion of patients who experienced at least one flare (58% versus 78%; p=0.02).8 However, in this study the top-down regimen consisted of only three infliximab infusions, with additional infusions only in cases of clinical deterioration (as was standard practice at the time of the study) rather than scheduled maintenance therapy (as is standard practice now). Furthermore, the step-up regimen allowed the introduction of infliximab, potentially reducing the differences in outcomes between study groups.7
The benefits of early intervention with anti-TNF in CD are supported by a systematic review and meta-analysis of 16 studies assessing early biologic (<2 years’ disease duration or initiation of biologics prior to immunosuppressants) or late biologic/conventional therapy use (>2 years’ disease duration, conventional management, or step-up therapy) in a total of 18,471 patients. Rates of clinical remission and endoscopic healing were significantly higher, and rates of relapse significantly lower, in patients receiving early biologic therapy versus those receiving late biologic therapy or conventional treatment (Figure 1).9
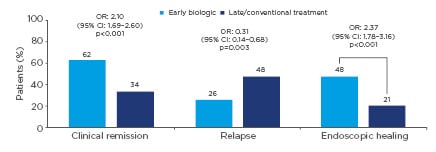
Figure 1: Clinical and endoscopic outcomes of early biologic therapy versus delayed or conventional treatment from a systematic review and meta-analysis of 16 studies in Crohn’s disease.9
CI: confidence interval; OR: odds ratio.
However, the evidence to support early intervention with anti-TNF is very limited in UC. Only preliminary data are available that are indicative of a benefit of this strategy, with further evidence needed to fully understand its role in the management of UC. There is a rationale for inducing early mucosal healing with anti-TNF in UC, as demonstrated by a post hoc analysis of infliximab-treated patients with UC in the Phase III ACT-1 and ACT-2 studies (N=466).10 Amongst these patients, achievement of mucosal healing (Mayo endoscopic subscore of 0 or 1) at Week 8 was significantly associated with improvements in reducing time to colectomy (p=0.0004) and increasing rates of symptomatic remission and corticosteroid-free symptomatic remission (p<0.0001) up to 54 weeks.
The benefit of earlier introduction of biologics is also supported by a retrospective study of Korean patients with UC analysed by year of diagnosis (Cohort 1: 1977–1999 [n=704]; Cohort 2: 2000–2006 [n=979]; Cohort 3: 2007–2013 [n=1,119]).11 In this analysis, patients in Cohort 3 had the shortest time between diagnosis and anti-TNF initiation, and this was paralleled by a decreased colectomy rate versus the other cohorts.
Furthermore, results of the UC SUCCESS study (n=231) demonstrated that infliximab in combination with azathioprine was superior to azathioprine monotherapy in patients with moderate-to-severe UC despite corticosteroid treatment.12 In this study, infliximab combination therapy was associated with significantly higher rates of steroid-free remission (39.7% versus 23.7%; p=0.032) and mucosal healing (62.8% versus 36.8%; p=0.001) versus azathioprine monotherapy. These data add to the growing body of evidence supporting the clinical decision to introduce anti-TNF earlier in the treatment pathway with the potential to improve patient outcomes.
However, these studies have several limitations: they were not conducted specifically in patients with early UC (which may be considered to be <3 years’ disease duration), and they did not assess the impact of early versus delayed intervention with anti-TNF. To understand the role of early intervention with anti-TNF, prospective, interventional studies in patients with early UC are required.
Barriers to Early Intervention with Anti-TNF in Ulcerative Colitis
For early intervention with anti-TNF to become part of routine clinical practice, so that patients with UC can benefit from improved clinical outcomes, patients suitable for this management approach should have their patient journeys optimised. However, a number of barriers exist that may limit the use of early intervention in clinical practice.
Firstly, it can be challenging to identify the patients who may benefit most from early intervention with anti-TNF. Patients with risk factors for severe inflammation or an unfavourable disease course could benefit the most from early intervention with anti-TNF, while those with mild-to-moderate UC may be more suited to a step-up approach, as it is always important to consider the benefit–risk ratio of treatment.13 The difficulty is in identifying the patients who may require early intervention to improve outcomes, and making an appropriate and timely referral so that accurate assessment of the severity of the disease and presence of risk factors can be made and intervention be optimised.
A number of risk factors that lead to a more complicated disease course in UC (i.e., a need for surgery and the development of colon cancer) and proximal disease extension have been identified.14 These include young age at diagnosis, male sex, need for steroids at diagnosis, delay in diagnosis (>6 months), family history factors, severe disease activity at diagnosis, extensive colitis, and concurrent primary sclerosing cholangitis (Figure 2). However, these risk factors remain to be validated in the context of selecting patients for early intervention with anti-TNF.
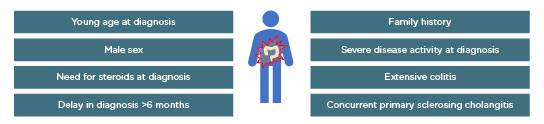
Figure 2: Risk factors for complicated ulcerative colitis disease and/or proximal disease extension.14
Another important barrier to early intervention with anti-TNF is the challenge of early diagnosis of UC. A European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) survey of 4,670 patients with IBD (33% of whom had UC) found that 45% of patients had not received a confirmed diagnosis within 1 year of symptom onset, and 17% had not received a diagnosis within 5 years.15 Delays in referral to a specialist are a key challenge in early diagnosis of UC, with 30% of patients in the EFCCA survey not having seen a specialist within 1 year of symptom onset.15 Other challenges in the diagnostic pathway include the lack of a single noninvasive diagnostic test for UC and symptoms that overlap with other diagnoses (including common conditions such as haemorrhoids and diverticular disease).
In CD, a ‘Red Flags’ index has been developed, which aims to reduce diagnostic delay by identifying early signs and symptoms that predict CD diagnosis.16 Presence of these factors could be used to identify patients with possible CD who should be referred to a specialist for further evaluation. Development of a similar tool for UC could help to reduce delays in referral to a specialist and support early diagnosis.
An additional barrier to early intervention with anti-TNF is the misperception that colectomy is a cure for UC with a lower risk of side effects than pharmacological intervention, and that disease progression is less of a concern because a curative procedure such as colectomy is available.
Thirty-year results of a survey of patients with UC who underwent ileal pouch–anal anastomosis surgery between 1981 and 2000 at a single centre (n=1,895) showed that 30–50% of patients experienced improved quality of life (based on seven domains: social activity, work around home, family relationships, travel, sports, recreation, and sexual life).17 However, 80% of patients also reported pouchitis, and 42% experienced daytime incontinence after surgery. Furthermore, a systematic review and meta-analysis of 28 studies reporting outcomes of colectomy found that 39% of patients experienced long-term (>30 days) postoperative complications, including pouchitis (29%); faecal incontinence (21%); small bowel obstruction (17%); and pouch failure, loss, or excision (5%).18 These studies suggest that there remains a need for optimised pharmacological intervention in the management of UC and that colectomy may not be an ideal solution.
Cost is another major barrier to early intervention with anti-TNF. A survey by the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) group that assessed dermatologists’ and rheumatologists’ perspectives on currently available therapies found that cost was the most common limitation preventing initiation of biologics (48.8% of dermatologists and 45.9% of rheumatologists) and one of the top three most common limitations preventing continuation with biologics (21.2% and 19.1%, respectively).19 It was highlighted during the discussion, however, that biosimilars reduce the cost of anti-TNF agents, and therefore have the potential to improve access to these therapies earlier in the disease course.
Treatment Goals in Early Ulcerative Colitis
If the decision is taken to treat earlier in the disease course in patients with UC, it is critical to identify an appropriate treatment goal. According to the 2015 recommendations from the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) programme, UC treatment goals should be clinical/patient-reported outcome remission (defined as the resolution of rectal bleeding and diarrhoea or altered bowel habit) and endoscopic remission (defined as a Mayo endoscopic subscore of 0 or 1). Histological remission was included as an adjunctive goal, but was not a primary target because of a lack of evidence of clinical utility (Figure 3).20

Figure 3: Current and potential future treatment goals in ulcerative colitis.20
PRO: patient-reported outcome; STRIDE: Selecting Therapeutic Targets in Inflammatory Bowel Disease.
However, accumulating evidence supports the use of histological remission as a treatment goal. Histological remission is a more stringent endpoint than endoscopic remission, as demonstrated by a prospective study of patients with UC in clinical and endoscopic remission (N=96), in which 13% and 43% of patients with a Mayo endoscopic subscore of 0 or 1, respectively, had histologically active disease.21 Histological activity (Geboes score ≥3.1) was significantly associated with clinical relapse over 12 months (p=0.011). In contrast, the clinical relapse rate was similar regardless of the degree of endoscopic remission (Mayo endoscopic subscore of 0 or 1; p=0.894). Histological activity was also associated with an increased risk of colorectal neoplasia in a meta-analysis of six studies (N=1,443; odds ratio: 2.58; 95% confidence interval: 1.49–4.46); this is therefore another important factor in the clinical decision process.22
Studies have shown histological remission to be an attainable target in patients treated with conventional therapy or biologics. In a randomised, double-blind study of patients with mild-to-moderate UC (N=343) treated with budesonide or mesalamine, 47.5% and 58.4% of patients, respectively, achieved histological remission (histological index ≤1) at Week 8.23 In a population with moderate-to-severe UC treated with adalimumab (N=34), 17.6% of patients achieved histological remission (Geboes score ≤3.0) at Week 8, and this increased to 31.0% (per protocol population) or 26.5% (intention-to-treat population) at Week 52.24 However, there is no consistent, validated definition of histological remission in UC,25 and this contributes to another barrier to the adoption of histological remission as a treatment goal in clinical practice.
The faculty highlighted the importance of individualised treatment targets in the management of UC. In patients with highly refractory disease, histological remission may be too stringent a target, potentially leading to inappropriate repeated switching between therapies when the target is not met, even in patients who have acceptable disease control. Shared decision-making is also important when setting treatment targets, to ensure patients achieve the goals that are most important to them.
Concluding Remarks
UC is increasingly seen as a progressive disease, similar to CD, and early intervention may prevent progression and improve long-term clinical outcomes. In CD, a growing body of evidence supports the use of early intervention with anti-TNF to improve long-term outcomes. In UC, data suggest that early mucosal healing is associated with improved longer-term outcomes, but prospective, interventional studies assessing early intervention with anti-TNF are lacking. A number of challenges exist in the management of UC, including delayed diagnosis, misperceptions about the disease and its management, and the cost of biologics. Overcoming these barriers will play a key role in improving patient access to anti-TNF in early UC and improving treatment outcomes. Treatment goals are a key consideration in the management of early UC. While increasing evidence supports histological remission as a treatment target, it remains challenging to attain in clinical practice, particularly in patients with highly refractory disease. Alongside selection of an appropriate treatment target, the key to successful management of UC is to optimise therapy for each patient with the right drug early in the disease course.








