Meeting Summary
The current treatment strategy for patients with inflammatory bowel disease (IBD) aims to enable physicians to deliver optimal care and to improve the role that patients play in treatment decisions. The multidisciplinary team (MDT) approach integrates the patient’s perspective and sees the discussion of treatment options with both gastroenterologists and surgeons as early as possible. The MDT approach is also vital in managing the risk of IBD and cardiovascular-related comorbidities in patients with psoriasis (PsO) and psoriatic arthritis (PsA), where selection of appropriate medication may affect both the rheumatic condition and the associated comorbidity. Close interdisciplinary interactions between gastroenterologists, rheumatologists, and/or dermatologists are vital, and the ensuing knowledge transfer facilitates the provision of optimal patient care. Personalised medicine will have a profound impact on future treatment algorithms in IBD and other chronic inflammatory conditions. Owing to the complexity of these diseases, a novel approach is urgently needed that will aggregate data from multiple systems and integrate it into a so-called ‘IBD interactome’. This may help identify and target the key molecular components responsible for inflammation. Future treatment practices will also address the psychosocial aspects of IBD by empowering patients and integrating their perspective into the shared treatment decision-making process early on.
Optimising Multidisciplinary Care in Inflammatory Bowel Disease
Doctor Krisztina Gecse and Professor Antonino Spinelli
In an MDT approach to the management of IBD, the treatment plan is overseen by gastroenterologists and surgeons, with additional input from other MDT members including psychologists, stoma nurses, dermatologists, rheumatologists, IBD nurses, pathologists, and radiologists (Figure 1). Early involvement of the surgeon is particularly important in cases of acute severe ulcerative colitis (UC), a severe flare, experienced by approximately 20–25% of patients with UC, that requires hospitalisation and immediate intensive medical and surgical monitoring.1-4
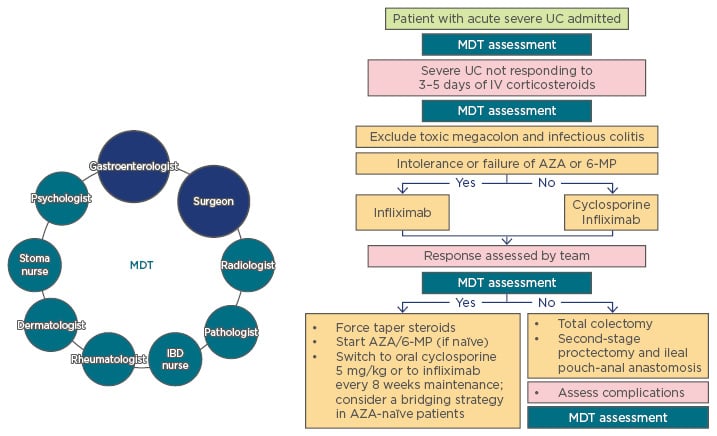
Figure 1: Multidisciplinary team involvement in treatment decisions for acute severe ulcerative colitis.
Gastroenterologists and surgeons comprise the core of the MDT, which also includes the IBD nurse, psychologist, rheumatologist, stoma nurse, dermatologist, pathologist, and radiologist.
AZA: azathioprine; IBD: inflammatory bowel disease; IV: intravenous; MDT: multidisciplinary team; MP: mercaptopurine; UC: ulcerative colitis.
Adapted from van Assche et al.5
Currently, the typically applied definition of acute severe UC follows the Truelove and Witts criteria: frequent bloody diarrhoea (≥6 times a day) accompanied by signs of systemic toxicity, such as pulse rate >90/minute, body temperature >37.8°C, haemoglobin <105 g/L, erythrocyte sedimentation rate >30 mm/h, or C-reactive protein >30 mg/L.6 After admission, a daily clinical assessment is performed to facilitate early recognition of complications and negative prognostic factors which help predict the need for surgical intervention. Surgery becomes mandatory if complications occur, such as toxic megacolon, perforation, or severe colorectal haemorrhage.7
First-line rescue therapy with a 5-day intensive intravenous regimen of steroids has a remission rate of 73%.2 However, after 3 days of intensive treatment, patients with either frequent stools (>8/day), and/or elevated C-reactive protein (>45 mg/L) need to be identified for further intervention. Failure of first-line and subsequent rescue therapies increases the risk that surgery will be required.8 The likelihood of colectomy following the initial rescue therapy has been analysed in patients undergoing second-line rescue treatment with cyclosporine A or infliximab.9 In this study, no difference was found between the two regimens; however, another retrospective study has shown that accelerated dosing of infliximab results in a significantly lower colectomy risk compared with standard dosing.9,10 Surgery is indicated in most patients who do not respond to standard rescue treatments. Daily clinical assessment is performed after the initiation of therapy. The timing of surgical intervention is critical because prolonged, ineffective systemic treatment can lead to a deterioration in general health and contribute to postoperative morbidity and mortality.7
It is important to conduct a surgical consultation promptly after patient admission and to intimately involve the patient in this consultation and subsequent treatment decisions, as this helps to manage patient expectations. In the setting of acute UC, patients should be informed about available medical and surgical options and their respective prognoses as early as possible. The decision regarding surgery timing should be shared by both gastroenterologists and colorectal surgeons.7,11 Colectomy can and should be considered as the first option in certain subsets of patients, such as when the patient has a contraindication to steroids and/or biologics, when complications arise (e.g., toxic megacolon or perforation), or if there have been recurrent hospitalisations for acute severe UC. In addition, patient age may be a factor in selecting the optimal treatment approach.7
Delaying surgery for patients who fail to respond to rescue therapy is associated with an increased risk of postoperative complications. A prospective study of 80 patients undergoing subtotal colectomy for acute severe UC in the UK between 1994 and 2000 showed that a prolonged preoperative hospital stay is associated with higher short and long-term postoperative morbidity.12 Similarly, a retrospective analysis of a nationwide database from the USA included 7,108 patients who underwent subtotal colectomy from 1995–2005.13 In this study, prolonged preoperative hospital stay was associated with both higher postoperative morbidity and mortality.13
The recommended surgical procedures in acute UC are colectomy and ileostomy, with the rectum left in situ.11,14 A laparoscopic approach is at least as safe as open surgery, but it has the advantages of a decreased rate of postoperative infections and abdominal abscesses and is associated with a shorter hospital stay. It is also associated with a better-preserved body image and reduces the risk of lower bowel obstruction.7,11
In conclusion, there are several time points at which an MDT meeting should be part of the treatment decision process (Figure 1). Gastroenterologists and surgeons should be consulted as soon as the patient with acute severe UC is admitted to the hospital; further meetings may be required after first-line rescue treatment or on Day 3 of steroid treatment, and when assessing the response to second-line rescue treatment.5 Any complications that arise should be assessed by the whole MDT, and, if necessary, post-second-line daily evaluations by the gastroenterology team should also include daily consultations with the surgical team. In conclusion, the patient should be included in setting treatment goals in UC as well as other IBD indications. Furthermore, the patient’s quality of life after surgery should be taken into account when assessing treatment success.
Managing Comorbidities: Lessons Learned from Rheumatology
Doctor Frank Behrens
In rheumatologic diseases, associated diseases and comorbid conditions occur in parallel with the core disease, and may drive treatment decisions and necessitate a MDT approach to management. The number of surgical interventions for rheumatic diseases, including rheumatoid arthritis, has reduced dramatically since the introduction of biologics to the treatment landscape.15 With the increasing number of therapy options currently available to patients with arthritis, the physician needs to carefully choose interventions that will not only address the signs and symptoms of arthritis, but also treat any associated disease.
PsO is one of the most common skin diseases in Central Europe and 30% of patients with PsO are also affected by PsA.16 PsA is associated with structural damage to the joints, as well as with systemic inflammation. The disease is highly heterogeneous, and symptoms may include swollen, tender joints, enthesitis, ankylosis, nail PsO or nail impairment, and skin manifestations.17 In addition, patients with PsO are at risk of developing IBD and often have cardiovascular and metabolic comorbidities.
A nationwide register study in Denmark showed that patients with PsO had a higher incidence of Crohn’s disease (CD) compared with the general population. Adjusted incidence risk ratios were 1.28 (95% confidence interval [CI]: 1.03–1.59) for mild PsO, 2.56 (95% CI: 1.87–3.50) for severe PsO, and 3.42 (95% CI: 2.36–4.95) for PsO with concomitant PsA. Similarly, the adjusted incidence risk ratios of UC were 1.49 (95% CI: 1.32–1.68), 1.56 (95% CI: 1.22–2.00), and 2.43 (95% CI: 1.86–3.17), respectively.18 Two prospective Nurse’s Health Studies of 174,476 women in the USA showed an 8-fold increased risk of CD in women with PsO and PsA (Table 1).19 It should be noted that the presence of concomitant PsA increases the risk of IBD >3-fold and a large proportion of patients with spondyloarthropathies (PsA, ankylosing spondylitis, reactive arthritis) have inflammatory lesions of the bowel that may not be typical of IBD, but are indicative of chronic inflammation.20,21 Notably, although the occurrence of intestinal inflammation has been linked with the use of certain medications (e.g., corticosteroids), other studies suggest that it is caused by the rheumatic condition itself.22
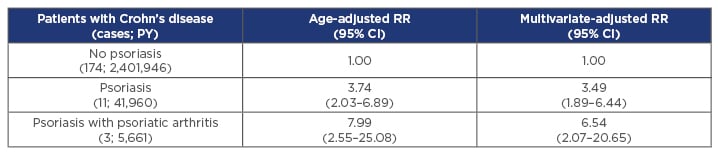
Table 1: Patients with psoriasis have a significantly increased risk of developing Crohn’s disease.
Results of a prospective cohort study from the USA (N=174,476) indicate that women with PsO with or without PsA have a significantly increased risk of developing Crohn’s disease compared with the general population.
CI: confidence interval; PsA: psoriatic arthritis; PsO, psoriasis; PY: patient years; RR: relative risk.
Adapted from Li et al.19
Awareness of possible inflammatory comorbidities should be maintained when making treatment decisions. For example, therapies targeting interleukin-17 have demonstrated efficacy in PsO, but not in IBD, whereas anti-tumour necrosis factor (TNF) therapies have shown activity in both PsO and IBD.23-25 Thus, if a patient with PsO shows signs of inflammation in the gut that may indicate early stages of IBD, an anti-TNF agent may be more appropriate.
Psoriatic disease is also associated with an increased risk of cardiovascular disease. Increased incidence of major adverse cardiac events, myocardial infarction, cerebrovascular disease, atherosclerosis, and ischaemic heart disease in patients with PsO results in a reduced life expectancy compared with the general population.26-28 A recent meta-analysis of five studies, totalling 49,795 patients with PsO, demonstrated that some of the available treatments for PsO may reduce the risk of adverse cardiovascular events.29 Specifically, treatment with TNF inhibitors was associated with a significantly lower risk of cardiovascular events compared with topical or phototherapy (relative risk: 0.58; 95% CI: 0.43–0.77; p<0.001) or with methotrexate treatment (relative risk: 0.67; 95% CI: 0.52–0.88; p=0.003).29 The new European guidelines on the treatment of rheumatic conditions warn clinicians of the higher risk of cardiovascular disease in patients with rheumatoid arthritis and other inflammatory joint diseases and place the responsibility for cardiovascular risk management on the rheumatologist.30
The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has recently reviewed the data on the most pertinent comorbidities in patients with PsO or PsA and their effect on treatment, and provided detailed treatment recommendations.31 In line with these recommendations, individual treatment decisions should be driven by available efficacy data, both in the core disease and in possible concomitant conditions; concomitant conditions can be identified by a careful assessment of symptoms beyond those associated with the core disease. Increasing the predicted lifespan for patients with chronic rheumatic diseases to equal that of the general population and beyond should be used as the measure of treatment success.
New Multidisciplinary Approaches: What Does the Future Look Like?
Professor Claudio Fiocchi
The last two decades have been spent in constant search for better treatments for immune-mediated inflammatory disorders; however, rather than achieving a cure, we have merely increased the number of therapeutic options available. New therapies are designed based on increasing knowledge of disease pathogenesis and aimed against carefully selected targets, including molecules that regulate interactions between leukocytes and endothelial cells at sites of inflammation, molecules that modulate the migration of T cells in the blood, and intracellular signalling components, such as Janus kinases.32,33
The introduction of anti-TNF therapies revolutionised the management of several autoimmune and chronic inflammatory diseases: rheumatoid arthritis, PsA, PsO, ankylosing spondylitis, CD, and UC. Nevertheless, up to 40% of patients do not respond to initial treatment with biologics (infliximab, adalimumab, golimumab, vedolizumab, tofacitinib), and up to 50% experience reduced responses over time.25,34 Furthermore, of the biologic therapies that have been carefully designed in past decades, many failed to work in the clinic.35 The community seems to have reached a plateau of options and may be at the stage where creating a new drug based on current understanding is not the best way forward; instead, future drug discovery should be based on comprehensive systems biology approaches.36
Chronic inflammatory diseases of unknown aetiology are highly complex and are often driven by a combination of factors. Intestinal inflammation in IBD involves an interplay between various subtypes of immune and non-immune cells, and may be influenced by the composition of gut microbiota and other environmental factors, such as diet and smoking (Figure 2).35 It is known that the exposome (external environment), genome (patient’s genetic material), immunome (pattern of immune response), and microbiome (composition of gut microbiota) influence each other in many positive and negative ways, and no two patients are alike.37 Previous studies have generated a wealth of genomic, epigenomic, transcriptomic, proteomic, metabolomic, and microbiome data, which may be used in future ‘network medicine’ initiatives.38 These systems biology approaches will involve studying the molecular interactions between each of the omes, producing an overall disease network: the so-called ‘IBD interactome’. New concepts and tools are needed to define this IBD interactome, build a comprehensive molecular map of IBD, and identify the key molecular drivers of inflammation.35
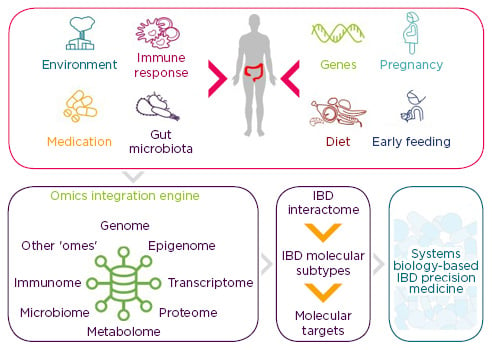
Figure 2: New multidisciplinary approaches to inflammatory bowel disease.
Future systems biology approaches will integrate data obtained through analysis of genome, epigenome, etc., producing an overall disease network. Other ‘omes’ are used in the sense that there might be still unrecognised omes that are relevant for IBD.
IBD: inflammatory bowel disease.
Adapted from Stern et al.,39 Ananthakrishnan et al.,40 and Schultze.41
The number of possible molecular interactions in healthy and diseased tissues is immense, and big data integration methods need to be developed to handle this information effectively.42 In addition, data obtained from single tissues may miss important regulatory interactions that are responsible for the pathogenesis of IBD. To reproduce this complexity of IBD, biological omics data derived from multiple sources will need to be integrated to construct regulatory network models.43 Novel software packages are available to assist with the modelling and visual display of complex omics data, including Cytoscape, VisANT, Pathway Studio, ProViz, and others.35 Further optimised bioinformatic tools are continuously being developed with the aim of simplifying the identification of central hubs (central regulatory molecules) within these extensive datasets.
Identifying the key components of the ‘IBD interactome’ will improve the specificity of the approach to disease subtyping, biomarker discovery, and drug repurposing. Importantly, it will enable a molecular classification of patients that is essential for the development of individualised treatment algorithms and for optimising the efficacy of existing treatment options.
Panel Discussion Precision Medicine: Current and Future Considerations
Professor Claudio Fiocchi, Doctor Krisztina Gecse, Professor Antonino Spinelli, Doctor Frank Behrens, Doctor Luisa Avedano
A panel discussion followed the presentations, allowing the faculty to share insights into current and future considerations for treating IBD, from the perspectives of the patient, gastroenterologist, colorectal surgeon, and rheumatologist.
Considering unmet needs from the patient’s perspective, Dr Avedano, CEO of the European Federation of Crohn’s & Ulcerative Colitis Associations (EFCCA), explained that it is important for patients with IBD to achieve a better balance between quality of care and quality of life. Sometimes, an excellent approach to the disease itself does not offer the improvement that the patient had hoped for. Additionally, IBD is a complex chronic disease that has a large psychosocial component. Often, the diagnosis of IBD has an enormous psychological impact on the patient, and physicians need to adopt a step-by-step approach to providing the patient with the right information at the right time, giving the patient time to assimilate the information to prepare for treatment discussions.
In this setting of chronic disease, the role of IBD nurse should not be underestimated. Dr Avedano mentioned that many nurses play the role of psychologist and offer advice on anxiety management and nutrition. In the context of interdisciplinary case management, MDT consultations may also help to decrease the patient’s anxiety. Different specialists often express slightly different views, and the possibility to speak to the whole team at once may bring patients on board faster and prevent confusion.
Dr Gecse and Prof Spinelli emphasised the importance of shared decision-making and involving the patient as early as possible, especially when raising the potential need for surgery, which may be harder for the patient to accept than non-invasive treatment options. Prof Spinelli noted that precision surgery in IBD can and should follow an individualised, patient-centric approach. The future of IBD surgery is to achieve clinical effectiveness while preserving the patient’s body image and quality of life.
Another aspect of individualised care lies in the variety of clinical presentations and molecular profiles of chronic immune-mediated inflammatory diseases. Prof Fiocchi emphasised that the same therapy or environmental factors may affect individual patients differently. Furthermore, diseases evolve on the molecular level, causing changes that may alter treatment efficacy. Dr Behrens remarked that many observations can be extrapolated across different inflammatory conditions; for example, the impact of environmental factors and microbiota observed in the pathogenesis of IBD may also be applicable to rheumatic diseases.
Conclusion
In conclusion, a personalised, patient-centric model will shape the future of IBD care, and a MDT approach will contribute to the clinical success of new therapies. The future treatment landscape will incorporate knowledge derived from integrating data in a systems biology approach. Finally, patient empowerment should be addressed by involving patients directly in treatment decision-making and by addressing psychosocial aspects of IBD early on.








