Meeting Summary
The epidemiology of diverticular disease (DD) is changing, with an increasing prevalence in younger patients from Europe and the USA, and changing disease patterns also seen in Asian populations. This epidemiological shift has substantial implications for disease management policy and healthcare costs. Most (75–80%) patients with diverticulosis never develop symptoms. Around 5% develop acute diverticulitis or other complications, while 10–15% develop symptomatic uncomplicated DD (SUDD) with symptoms resembling irritable bowel syndrome (IBS). However, most available guidelines highlight the importance of diverticulitis, with less emphasis on and often limited discussion about SUDD and its management. Recent data suggest an important relationship between gut microbiota and DD, including SUDD. In healthy individuals, the gut microbiota exists in harmony (eubiosis); in individuals with disease, quantitative and qualitative changes in microbial diversity (dysbiosis) may adversely influence colonic metabolism and homeostasis. Addressing this imbalance and restoring a healthier microbiota via eubiotic or probiotic therapy may be of value. In SUDD, clinical benefit has been seen with the use of rifaximin, which acts by multiple mechanisms: direct antibiotic activity, a modulatory eubiotic effect with an increase in muco-protective Lactobacillus and Bifidobacterium organisms, and anti-inflammatory effects, among others. Clinical studies have demonstrated symptom improvement and reduction in complications in patients with SUDD, with a favourable safety and tolerability profile and no evidence of microbial resistance. Evidence for other agents in DD is less robust. Mesalamine is not effective at preventing recurrence of acute diverticulitis, although it may provide some symptom improvement. At present, there is insufficient evidence to recommend the use of probiotics in SUDD symptom management.
Introduction
Colonic diverticula are herniations of the colonic mucosa and submucosa through the colonic wall. Colonic diverticulosis is common with increasing age, and while most individuals with diverticulosis remain asymptomatic, 10–35% will develop symptoms of DD.1 Of these patients, 85–90% will develop symptomatic SUDD and 10–15% will develop acute inflammatory and possibly sub-colitic changes, chiefly acute diverticulitis, with or without complications, which include abscesses, fistulae, and perforation (Figure 1).
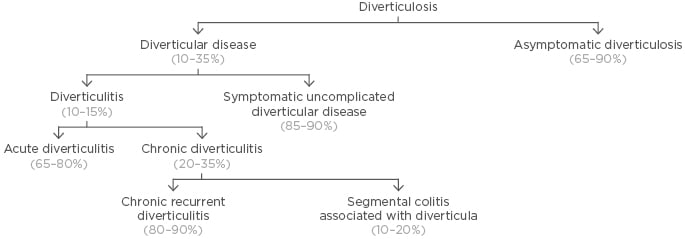
Figure 1: Taxonomy and relative frequency of diverticular disease.
Adapted from Scarpignato et al.1 and Cuomo et al.2
SUDD is defined as persistent and recurrent abdominal symptoms attributed to diverticula in the absence of obvious inflammatory changes in the colonic mucosa. The cardinal symptom of SUDD is abdominal pain or fullness, often accompanied by bloating and bowel habit abnormalities. There is a wide overlap between SUDD and IBS, both in terms of symptoms and management. This overlap is well recognised, although patient profiles (generally SUDD patients are older) and clinical features, especially those related to abdominal pain severity, which is substantial in SUDD; location of the pain (more diffuse in IBS, but localised to the lower left quadrant in SUDD); and duration of pain (>24 hours in SUDD) may help distinguish between SUDD and IBS. Other parameters, such as faecal calprotectin levels (sometimes elevated in SUDD but rarely in IBS), are also helpful.1,2 An additional consideration is that IBS-like symptoms may emerge after acute diverticulitis.3
A number of published guidelines or consensus statements on the diagnosis and treatment of DD are available from Europe2,4-8 and the USA.9,10 There are no specific guidelines from Asia, although discussion of DD in Asia is included in guidance from the World Gastroenterology Organisation (WGO).11 Most guidelines focus principally on acute diverticulitis (treatment and primary and secondary prevention), and specific discussion on diverticulosis and SUDD is often lacking.12 Notable exceptions are the most recent consensus statements from Italy and Poland.2,7,8 SUDD is not discussed in the current USA guidelines because the currently available evidence was considered limited.10
Epidemiological Aspects of Diverticulosis and Diverticular Disease
Diverticulosis shows an age-dependent distribution, with a prevalence reaching 60% in individuals >80 years old.13 However, epidemiological data suggest an increasing prevalence of diverticulosis and DD in younger patients.14 In Italy, recent data indicate an increase in hospitalisation for acute diverticulitis from 2008–2015 (from 39 to 48 per 100,000 inhabitants) and while hospitalisation rates remained relatively stable in patients aged >70 years, a significant increase in hospitalisation rates due to acute diverticulitis in younger patients (including those aged 18–39 years) was observed.15 Other Italian data also report increasing numbers of admission due to complicated disease in younger patients.15,16 A corresponding increase in health-system costs has also been seen, and although much of the cost burden is due to complicated disease in older patients (especially those requiring surgical care), the hospitalisation costs associated with uncomplicated disease are also substantial.16
Recent colonoscopy surveillance data also show increasing prevalence of diverticulosis in some Asian countries (Singapore and Japan).17,18 There is substantial regional variation in the prevalence of diverticulosis across Asia, with higher rates in the Philippines, Singapore, Hong Kong, and Japan (ranging from 25–35%) and a far lower prevalence in India, China, and South Korea (1–3%).17 Right-sided disease remains the predominant phenotype in Asia.17 In Singapore, the prevalence of right-sided diverticulosis increased from 15% to 25% between 2006 and 2016; left-sided diverticulosis increased from 5% to 10%, and pan-colonic diverticula increased from 3% to 5%.17 In contrast, in India, the prevalence of diverticulosis remained low and relatively stable between 2010–2015.17 Singapore is a multi-ethnic society and an important observation is that increased prevalence of diverticulosis was seen across all three major ethnic groups: Chinese, Malay, and Indian.17 Although speculative, it is possible that a rise in prevalence could follow the adoption of Western-style diets and the greater prevalence seen in Singapore, Hong Kong, the Philippines, and Japan may reflect this rather than any ethnic or genetic component. If this were the case, a concern is that, following any switch to such a diet, a higher prevalence may also arise in currently low-prevalence populations.
Data from Japan show that right-sided diverticulosis begins to occur before 39 years of age, with a prevalence of 4%, which increases at 40 years and 60 years of age to 10% and 16%, respectively. On the other hand, the prevalence of left-sided DD begins to increase from the age of 50 years, and progressively increases until beyond the age of 70 years.18 In this study, alcohol consumption was a risk factor for right-sided disease, while smoking was a risk factor for both right and left-sided disease.18 Another Japanese study found that left-sided and bilateral diverticulosis (but not right-sided disease) were associated with a higher risk of IBS.19
Pathophysiology Update
Studies have demonstrated increased intraluminal pressure and motility following provocative stimuli in patients with diverticula compared to healthy controls.20 In Western populations, this involves the sigmoid region, while in Asian patients these features are seen also in the ascending colon in patients with right-sided diverticulosis.20,21
While age-related weakening of the colonic wall is an important feature of diverticulosis, this involves only the descending colon.22 Minimal changes in colonic wall strength have been observed in the ascending colon in Western and Asian patients. While this may explain, in part, the left-sided predominance of diverticulosis in Western populations, the absence of weakening in the affected site in Asian patients highlights the role of other factors in disease mechanisms, including stool form and viscosity in different colonic segments, as well as genetic and dietary components.22 Other colonic tissue changes may also play a role; for example, altered extracellular matrix and collagen rearrangement predispose to increased rigidity and elastosis in the colonic wall.2,20
Pain is an important feature in SUDD. In patients with diverticulosis, an increase in the numbers and density of nerve fibres within the colonic mucosa is seen at affected sites. This is particularly evident in patients with SUDD, in whom increased nerve sprouting can also be demonstrated in the affected region, a feature less evident in asymptomatic disease.23 It is possible that this may underlie pain transmission in SUDD patients, although further studies are needed to support this hypothesis. Measurement of visceral sensitivity by rectal distention has found that patients with symptomatic disease have lower pain thresholds than asymptomatic patients, along with increased expression of neuropeptides (e.g., neurokinin-1) in the colonic mucosa in symptomatic patients.24
The Intestinal Microbiome
The role of the gut bacterial microbiota in health and potential changes contributing to DD is growing in importance. The putative role of microbiota in the pathogenesis of DD is also supported by evidence that most disease complications (e.g., inflammation, fistulae, and abscesses) are of bacterial origin. The relationship between gut microbiota and disease may be considered as that of eubiosis, when there is a healthy quantitative and qualitative balance between microbiota and host metabolism and immunology, or dysbiosis, when quantitative and qualitative changes to gut flora are associated with altered colonic metabolism and immune function. Colonic microbiota is inherently linked to diet, with different compositions in populations with a diet rich in red meat and fat compared with those receiving a high-fibre diet.25 Dietary fibre is an important source of energy for the gut microbiota and gut microbiota metabolise complex carbohydrates into short-chain fatty acids, which influence both mucus and antimicrobial peptide production.
Differences exist in the colonic microbiome in healthy subjects compared to that seen in patients with DD. For example, recent studies have shown that, compared with healthy subjects, an overgrowth of Aeromonas species (e.g., A. muciniphila) and higher relative abundance of Bifidobacterium are found in patients with acute diverticulitis or SUDD.26,27 Other studies have shown a higher diversity of Proteobacteria and higher levels of Actinobacteria in patients with acute diverticulitis compared to those with uncomplicated diverticulosis.28,29 Another recent study examined colonic mucosal biopsies and also the faecal microbiome in patients with diverticulosis or SUDD.30 Microbiome analysis showed that patients with diverticulosis had a microbiota enriched in Bacterioides and Prevotella (encompassing several groups of bacteria with proinflammatory properties), while patients with SUDD had depletion of a range of species (Clostridium cluster IV and IX, Fusobacterium, and Lactobacillus species) associated with anti-inflammatory pathways or production of muco-protective short-chain fatty acids. Biopsy comparisons found no differences in mucosal T cell or mast cell numbers, but a >70% increase in colonic macrophages was seen in patients with diverticulosis and SUDD (at affected and at distant sites).30 This suggests a potential role for mucosal macrophages as a marker for DD.
It must be remembered that these findings do not indicate causality, and other studies have found no associations between microbiota composition changes and disease.31 Most studies examining such associations are small, and dietary changes and treatment of DD may have contributed to changes in gut microbial ecology.
Controversies in Disease Management
Historically, antibiotics were considered a standard treatment for acute diverticulitis. However, data from two recent randomised clinical trials (RCT) have indicated little to no benefit from antibiotic administration in patients with acute uncomplicated diverticulitis.32-35 In the AVOD study,32 which compared observational management with or without antibiotics, there were no differences in reported abdominal pain or tenderness during inpatient care, and both groups had similar durations of hospital stay. At 12-month follow-up, similar rates of complications, such as abscess, perforation (1–2%), and recurrent diverticulitis requiring readmission (16%), were reported.32 In the DIABALO study,33 which compared parenteral followed by oral antibiotics for 10 days versus observational care alone, no differences in time to recovery were seen for the two groups. However, the length of hospital stay was shorter in the observation group (2 versus 3 days; p=0.006).33 Both groups had similar rates of complications, recurrent disease, and subsequent readmission at 6-month follow-up,33 with similar rates of recurrent or complicated disease at 2 years.34 While no significant differences in the need for sigmoid resection were found, a trend towards more elective surgery in the observation group was seen.34 A recently published systematic review and meta-analysis has concluded that antibiotic use is not associated with reductions in rates of major complications, disease recurrence rates, or surgical resection, although antibiotic use may be associated with a significantly shorter duration of hospital stay.35 These findings support the approach that antibiotics in patients with uncomplicated acute diverticulitis should not be used routinely, with selective use reserved for the treatment of those patients with complicated disease, severe infection/sepsis, or significant comorbidities. This is reflected in treatment recommendations in the current Dutch, Italian, German, and USA guidelines.2,5,6,10
The role of diet, including dietary supplements and foodstuffs to avoid, is frequently debated. Fibre assists in stool bulking and colonic motility and promotes the growth of beneficial colonic microbiota (e.g., Bifidobacterium and Lactobacillus species). For these reasons, a fibre-rich diet would seem to offer protective benefits against DD.36 However, the evidence suggests different associations between fibre and diverticulosis and fibre and DD. For example, in the USA, the Diet and Health Studies III–V found that a high-fibre diet and increased frequency of bowel movements were associated with greater prevalence of diverticulosis.37 In contrast, large prospective studies from the UK (EPIC-Oxford study)38 and the USA (Health Professionals Follow-up Study)39,40 have shown an inverse association between fibre intake and diverticular complications, in which high-fibre dietary intake is associated with reduced risk of hospitalisation for DD,38 symptomatic DD,39 and acute diverticulitis.40 These somewhat contradictory findings suggest that the underlying mechanisms (and the influence of fibre) in the development of diverticulosis may be quite different to those involved in subsequent diverticulitis development; it would seem that fibre is of benefit in patients with DD and SUDD. The most recent guidelines from the USA suggest that a fibre-rich diet or fibre supplementation may be beneficial in patients with a history of acute diverticulitis.10
The evidence base for the role of fibre supplements is relatively limited and is principally from older studies, many of which have substantial methodological limitations, which leads to difficulty in drawing firm conclusions.36 This was reflected in recent Italian guidelines, which concluded that fibre supplementation alone provides controversial results in terms of symptom relief for SUDD.2
While it has been proposed that certain foodstuffs (e.g., seeds, nuts, and popcorn) can predispose to DD, data from a large prospective cohort study show no increased risk of diverticulosis or DD; indeed, subjects with the greatest consumption of nuts or popcorn had significantly lower risk than those with lowest consumption.41 This was reflected in the most recent USA guidelines, which recommended against advising patients with a history of acute diverticulitis to avoid nuts and popcorn.10
DD shows seasonal variation, and this could reflect sunlight exposure and vitamin D levels. A USA study found that patients with acute diverticulitis had significantly lower levels of vitamin D than those with diverticulosis, with the lowest levels seen in patients with complications or recurrent diverticulitis.42 However, no direct causal relationship can be made and, at present, no recommendations regarding vitamin D supplements have been made.
Role of Rifaximin in the Management of Symptomatic Uncomplicated Diverticular Disease
Rifaximin is a poorly absorbed oral antibiotic characterised by non-systemic absorption and resultant high faecal concentration with broad antimicrobial activity (against Gram-positive and Gram-negative aerobic and anaerobic bacterial species).43 Rifaximin acts via multiple mechanisms relevant to SUDD. In addition to overt antibiotic activity, rifaximin has a modulatory eubiotic effect on bacterial species, as seen in animal models and human clinical and metagenomic studies that have demonstrated an increase in Lactobacillus and Bifidobacterium after rifaximin treatment.44,45 Anti-inflammatory effects are exerted via different pathways, including activity against proinflammatory gut microbiota.43,46 An important mechanism is due to the role of rifaximin as a gut-specific ligand for the human nuclear pregnane-X receptor (PXR), expressed primarily in the gastrointestinal tract. PXR activation is considered critical for maintenance of intestinal integrity and in downregulation of inflammatory responses triggered by the gut microbes and NFκB-mediated proinflammatory cytokine pathways. Rifaximin-PXR binding can contribute to this.46
The effects of rifaximin in SUDD have been investigated in prospective RCT (three open trials and one double-blinded study involving a total of 1,660 patients),47-50 and in a subsequent meta-analysis study.51 In most studies, rifaximin was given with fibre supplementation (principally glucomannan, a highly soluble fibre), with the comparator group receiving glucomannan monotherapy; rifaximin was given as 400 mg twice a day for 7 days every month for 12 months (Table 1). Across these studies, symptom improvement (i.e., the number of patients free of symptoms for the previous 6 months) ranged from 56.5–89.7% in patients receiving rifaximin plus fibre (27–34% higher than that seen in the comparator groups receiving fibre only). In a meta-analysis, Bianchi et al.51 pooled data from all four studies; at 1-year follow-up, 64% of patients treated with rifaximin plus fibre supplements were symptom-free versus 34.9% of patients receiving fibre alone. The pooled rate difference for complete symptom relief was 29.0% (95% confidence interval: 24.5–33.6; p<0.0001). This translates as a number needed to treat of 3.51 In these studies, 2.8% of individuals in the comparator group developed acute diverticulitis compared with 1.0% of those receiving rifaximin, with a pooled rate difference of -1.9% (95% confidence interval: -3.4–[-]0.57%) in favour of rifaximin (p=0.006) and a number needed to treat of 50.51
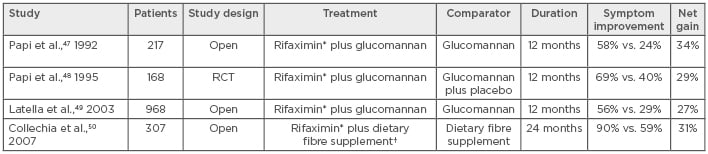
Table 1: Prospective randomised trials of rifaximin in symptomatic uncomplicated diverticular disease.
*Rifaximin 400 mg twice daily for 7 days each month for 12 months; †Dietary fibre supplementation of 20g per day.
RCT: Randomised controlled trials.
Rifaximin was safe and well-tolerated in these studies, with no significant differences in adverse events observed in the different treatment arms.51 A broader safety analysis of rifaximin in double-blinded, placebo-controlled trials in patients with DD or IBS found the safety and tolerability were comparable to placebo.52 In the TARGET3 study,53 in which patients with IBS received repeated courses of rifaximin, susceptibility testing of stool samples before and after each treatment course found no evidence of microbial resistance.
These data confirm the efficacy and safety of rifaximin as long-term cyclic (intermittent) therapy in SUDD. Differences exist as to how and where this can be used. For example, in the USA, rifaximin is licenced for IBS with diarrhoea and so is principally used in IBS patients with SUDD features. In contrast, in European countries it is used mainly in SUDD cases, although it may also have a role in clinically challenging (and non-constipated) IBS patients.
Secondary Prevention of Acute Diverticulitis
The role of mesalamine in prevention of acute diverticulitis recurrence has been extensively evaluated in large RCT, notably the DIVA and the PREVENT1 and PREVENT2 studies.54,55 In the DIVA study,54 patients with CT-confirmed acute diverticulitis were randomised to receive standard care plus either mesalamine, mesalamine and a probiotic (Bifidobacterium infantis 35624), or placebo for 12 weeks, with efficacy assessed using changes in a composite global symptom score involving 10 key gastrointestinal signs and symptoms. For the primary endpoint, there were no significant differences in recurrence rates between the three groups, although there was some evidence for symptom improvement with mesalamine.54 In the two PREVENT trials,55 comparing mesalamine (given for 2 years) with placebo, there were no differences in recurrence rates after 2 years (or in time to recurrence) in the mesalamine and placebo groups in either study. These data support the view that, although mesalamine may have a role in symptomatic treatment, this drug has no benefit in reducing disease recurrence, and this is reflected in the Italian, German, and USA guidelines.2,6,10
Studies evaluating the use of probiotics in the treatment and prevention of recurrent diverticulitis are of variable quality and are highly heterogenous regarding study treatments (e.g., probiotic alone or given with mesalamine) and treatment outcomes (although focussing primarily on symptom control).56-58 While this leads to difficulty in drawing firm conclusions, reported data indicate that the use of probiotics may have some role in symptom management, although, from an evidence-based perspective, no recommendations can be made.
Conclusion
Our understanding of diverticulosis and DD continues to evolve, with notable advances in biology, epidemiology, and therapeutic approaches. The role of rifaximin as a modulator of gut microbiota with potential anti-inflammatory activity holds promise as a therapy in patients with SUDD.








