Meeting Summary
The Janssen-sponsored symposium, entitled “20 20 Vision in IBD”, took place during the 15th Congress of The European Crohn’s and Colitis Organisation (ECCO) in Vienna, Austria, on 14th February 2020. Distinguished experts Prof Ghosh, Prof Atreya, and Prof Dignass illustrated the importance of the
IL-12/23 pathways in inflammatory bowel disease (IBD) management, and how these pathways
function in the larger picture of IBD pathogenesis. Prof Dignass presented the interim data from the STARDUST trial, the first treat-to-target, randomised clinical trial of adults with Crohn’s disease (CD)
using endoscopy at Week 16 as a decision point for dose adjustment of ustekinumab. The trial also
included an intestinal ultrasound (IUS) substudy to examine transmural disease activity and to assess the effectiveness of ustekinumab at achieving intestinal response and remission. The interim results showed that 75.0% and 87.0% of all patients receiving ustekinumab showed a CD activity index (CDAI) 70 response at Weeks 8 and 16, respectively. Furthermore, IUS response to ustekinumab was detected as early as Week 4 of treatment, and a clinically meaningful percentage of patients achieved transmural healing at Week 16. Prof Ghosh then highlighted how data from real-world studies mimic clinical trial results with ustekinumab in patients with CD, including patients who were previously exposed to one or more biologic treatment. The safety profile was also similar to already existing clinical trial results and the low risk of serious infections was highlighted.
Targeting the IL-12/23 Pathways in Inflammatory Bowel Disease
Professor Raja Atreya
Over the past years, the conceptual framework behind the pathogenesis of IBD has come into sharper focus. In healthy individuals, the intestinal mucosa encounters bacteria on a daily basis, but inflammation does not occur due to protection from the epithelial barrier and from cells that shield against over-reaction of the gut immune system. In patients with IBD, a multifactorial process takes place in the disease pathogenesis; there is impaired barrier function and thinning of the mucus layer, allowing luminal pathogens to enter the mucosa, activating immune cells and cytokines, and resulting in chronic inflammation.1,2 These evolving insights into the immunopathogenesis of IBD have enabled the development of targeted therapies.3
The structure and function of the gut-associated immune system incorporates activation of antigen-presenting cells upon encounter of an antigen, resulting in the induction of specific effector T cells. These effector T cells egress from the mesenteric lymph nodes into the blood, and then migrate into the intestinal tissue.4 This results in the local activation of T cells,4 which in turn activates a cascade of dysregulated immune responses, including aberrant cytokine secretion and the differentiation and proliferation of specific effector T cells that migrate into the intestinal tissue. This results in the heightened secretion of predominantly proinflammatory cytokines by effector T cells, such as TNFα, IL-5, IL-6, IL-13, IL-17, IL-21, and IL-22, leading to inflammation and IBD development.1,5
In the mesenteric lymph nodes, IL-12 activates the differentiation of T cells into proinflammatory Th1 effector cells.1,5 This differentiation can be inhibited via blockade of the IL-12/IL-12 receptor interaction, for example by the anti-IL12p40 antibody ustekinumab, that has been approved for the treatment of CD and ulcerative colitis (UC) patients.1,5 T-cell differentiation to a proinflammatory Th17 isoform is activated by IL-6 and TGF, and IL-23 is crucial for the survival and stabilisation of these cells.6-8 Therefore, blockade of the IL-23 pathway or the IL-23/IL-23 receptor interaction is an effective treatment strategy in IBD management.1,5 Several therapeutic agents block these pathways, including ustekinumab, or guselkumab, risankizumab, and mirikizumab, which are currently in development for the management of CD and UC.1,5
Exploring New Frontiers with ‘STARDUST’
Professor Axel Dignass
Treat-to-target is generally defined as a treatment strategy in which a target is preidentified and predefined, and treatment is optimised with regular monitoring and tight control until the target is achieved.9,10 This strategy is important because it incorporates a predefined target that is achievable via optimised therapy and personalised treatment plans. In IBD management, the goal of treat-to-target strategies is to achieve remission, endoscopic improvement, or endoscopic healing as appropriate, with regular monitoring necessary to reach the predefined targets.11 Treat-to-target goals in IBD, as defined in the 2015 STRIDE recommendations, include clinical remission, endoscopic improvement or healing, control of intestinal inflammation and normalisation of life, and avoidance of long-term bowel damage and subsequent disability.11,12 For patients with CD, these targets include resolution of abdominal pain, normalisation of bowel habits, and absence of ulceration. For patients with UC, the targets include resolution of rectal bleeding and normalisation of bowel habits, as well as a Mayo Endoscopic Subscore of 0 (optimal) or 1 (minimum). Cross-sectional imaging and biomarkers are not currently recommended as targets for CD or UC, but do serve as important surrogate markers for tight control of patients.11
Many previous and ongoing studies are focussed on tight control and treat-to-target approaches; for example, the CALM study is the first tight control study and included objective biomarkers of inflammation and clinical symptoms to drive treatment decisions.13 This approach led to superior endoscopic and clinical outcomes in CD when compared with symptom-driven care.13 The REACT 1 study compared the efficacy of an early combined anti-TNF treatment and an antimetabolite with that of conventional disease management for the treatment of CD.14 The results showed that this approach was not more effective than conventional management for controlling CD, though the risk of major adverse outcomes was lower. The ongoing REACT 2 treat-to-target study is examining whether the early use of combined therapy, with an antimetabolite and adalimumab, and treatment intensification based on ileocolonoscopic findings, will lead to better outcomes and disease modification compared with treatment escalation based solely on symptoms, with deep remission as the treatment target.
The STARDUST trial is the first treat-to-target, randomised clinical trial reporting results from adults with CD using endoscopy at Week 16 as a decision point for dose adjustment of ustekinumab. This study is examining whether a maintenance strategy based on early endoscopy and regular biomarker and clinical assessments, with subsequent adjustment of treatment and predefined ultrasound examinations to assess treatment response, is more successful in obtaining endoscopic improvement than a pragmatic maintenance strategy. The first results indicate that 75.0% and 87.0% of all patients showed a CDAI 70 response at Weeks 8 and 16, respectively (Figure 1).15 In addition, 83.0% and 83.9% of patients who were in clinical response were also in clinical remission at Weeks 8 and 16, respectively, with corresponding decreases in faecal calprotectin and C-reactive protein levels at both time points.15
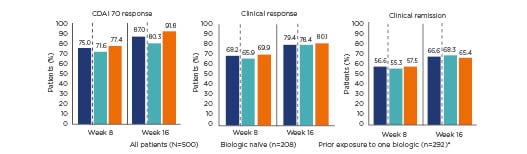
Figure 1: Crohn’s disease activity index 70 response, clinical remission, and clinical response at Weeks 8 and 16 of the STARDUST trial with ustekinumab, overall and by prior exposure to biologic treatment.15
*77.5% of patients with prior exposure to one biologic treatment experienced treatment failure.
CDAI: Crohn’s disease activity index.
All patients randomised to the treat-to-target arm were CDAI responders at Week 16; 58.2% of all patients showed improvements in simple endoscopic score for CD >25.0% at Week 16, with no differences between patients who were biologic-naïve and those who had prior exposure to one biologic treatment.15 Further results showed that 36.8% of all patients had an endoscopic response and 11.4% were in clinical remission at Week 16 (Figure 2).15
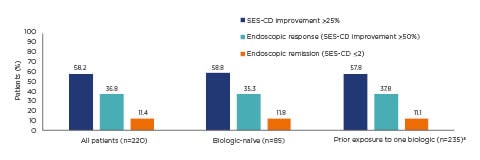
Figure 2: Endoscopic response and remission at Week 16 of ustekinumab treatment in the STARDUST trial.
Treat-to-target population: patients enrolled, treated, and randomised to the treat-to-target arm (n=220). The nonresponder imputation rule was applied for missing values or early termination.15
a77.5% of patients with prior exposure to one biologic treatment experienced treatment failure.
SES-CD: simple endoscopic score for Crohn’s disease.
The STARDUST trial also incorporates a substudy of IUS measures, which are comparable to MRI and CT in terms of sensitivity and specificity,16 to examine transmural disease activity and to assess the effectiveness of ustekinumab in achieving intestinal response and remission.17 These measures also offer insights into a possible relationship between IUS response and change of clinical and endoscopic parameters over time.17 Initial results from the IUS substudy show that the most affected segments were the ileum, in 66% of patients, and the colon, in 34% of patients, with increasing normalisation of bowel wall thickness over time in the most affected parts of the bowel.17
In conclusion, Prof Dignass emphasised that the STARDUST trial is the first interventional, multicentre, treat-to-target study in patients with CD, using endoscopy at Week 16 as a decision point for dose adjustment and exploring the use of IUS as a noninvasive tool to assess treatment response. After 16 weeks of induction treatment with ustekinumab, two-thirds of patients were in clinical remission and 37% of patients randomised to the treat-to-target arm (all CDAI 70 responders) were in endoscopic response, based on central readings. IUS response to ustekinumab was detected as early as Week 4 of treatment and a clinically meaningful percentage of patients achieved transmural healing at Week 16. These results underline the possible value of IUS as a tool to detect early response to treatment in CD. Furthermore, the safety and tolerability results were consistent with the known ustekinumab safety profile.
Sharpening Our Focus with Emerging Real-World Data
Professor Subrata Ghosh
In a discussion on the profile of ustekinumab in CD management, Prof Ghosh presented data from the IM-UNITI long-term extension trial, which show that 43.0% of patients receiving maintenance therapy with 90 mg ustekinumab every 8 weeks, and 38.0% of patients receiving the same dose of ustekinumab every 12 weeks, were in clinical remission at Week 152.18 Among anti-TNFα-naïve patients who were randomised from baseline, 53.9% receiving the maintenance dose of ustekinumab every 8 weeks and 50.9% receiving ustekinumab every 12 weeks were in clinical remission at Week 152.18 Furthermore, the data suggest that ustekinumab does not require concomitant treatment with immunomodulators to effectively treat patients with CD, a distinct difference when compared with anti-TNF therapies.19
However, it is also important to demonstrate that CD treatments are effective in the real-world setting. A national cohort study of 152 patients with CD, performed by the Belgian Inflammatory Bowel Disease Research and Development Group (BIRD), examined the long-term clinical effectiveness of ustekinumab. The results showed that, though the majority of patients had previously failed on one or more biologic therapies, a clinically meaningful proportion of patients showed steroid-free clinical responses to treatment as early as Week 8, as well as at Weeks 16 and 52, with low occurrences of
adverse events.20
In a Dutch national cohort study from the Initiative on Crohn’s and Colitis (ICC) Registry, which included 221 patients with CD, ustekinumab efficacy was maintained through Week 52 of treatment, with increases in clinical and steroid-free remission at Weeks 24 and 52.21 Of note, 73.0% of patients participating in the study had failed at least two prior anti-TNF therapies.21 Similar to previous clinical trials, maintenance therapy with 90 mg ustekinumab every 8 weeks was as effective as the 12-week dose in terms of corticosteroid-free remission at Weeks 24 and 52, without the need for concomitant immunomodulator treatment.21
A nationwide, real-world evidence study conducted in Finland (FINUSTE),22 consisting of a chart review from 17 centres and including 155 patients who received ustekinumab in 2017 or 2018, showed that ustekinumab treatment allowed for significant corticosteroid tapering through 1 year of treatment.23 More than 95.0% of these patients had a treatment history of one or more previous biologic treatment at baseline.22,23 Similarly, a Spanish Registry study (ENEIDA)24 showed that, although 96.0% of 305 patients participating in the study previously received anti-TNFα treatment, approximately 50.0% of the patients achieved a clinical response at Weeks 8 and 14 of ustekinumab treatment. Furthermore, approximately half of the patients achieved normalisation of faecal calprotectin levels and approximately one-third achieved normalisation of C-reactive protein levels at Weeks 8 and 14 of treatment.24 This study also revealed that the numbers of previous anti-TNFα treatments and cases of severe endoscopic activity were predictors of remission at Week 14 of treatment.24
An interim analysis of the Spanish SUSTAIN25 study, which examined the long-term effectiveness and safety of ustekinumab in 331 patients with active CD in real life, showed that ustekinumab discontinuation rates were low, and that ustekinumab was effective in real-world short and long-term use. Another study of 886 patients found that patients treated with vedolizumab and ustekinumab showed higher rates of treatment persistence than patients treated with anti-TNF agents.26 Patients treated with ustekinumab had the highest overall persistence rate.26
The safety profile of ustekinumab in the real world is comparable to findings from clinical trials. For example, data from the IM-UNITI LTE trial show that ustekinumab treatment resulted in low numbers of serious infections, comparable to the placebo group.18 Studies in special populations have showed that patients with CD treated with ustekinumab have a high seroconversion rate to the seasonal trivalent influenza vaccine, in contrast to the reduced seroconversion rate seen in patients treated with adalimumab. Therefore, patients receiving ustekinumab can be effectively vaccinated with the trivalent influenza vaccine.27 Furthermore, no severe neonatal and maternal complications occurred in female patients with IBD who were treated with ustekinumab or vedolizumab during pregnancy.28 Additional prospective evaluations regarding safety concerns of pregnancy outcomes in patients directly exposed to ustekinumab or vedolizumab are needed.28
In conclusion, Prof Ghosh emphasised that the current real-world evidence demonstrates the efficacy of ustekinumab in patients with CD, including patients who were previously exposed to one or more biologic treatment. The safety profile of ustekinumab in the real world is very similar to the safety data obtained from the UNITI and IM-UNITI clinical trials. Furthermore, the efficacy of ustekinumab is similar with both combination therapy and monotherapy, and has been shown to be safe in special populations, with high seroconversion rates in patients receiving influenza vaccines and no severe neonatal or maternal complications during pregnancy.








