Abstract
Clear or involved resection margins have significant bearings on the outcome of colorectal cancer cases. There are two aspects of resection margins: longitudinal and circumferential. Pathological staging for rectal and colonic tumour specimens is a useful tool for providing continuous feedback to surgeons and may serve to improve the quality of surgery and pathology reporting. It is expected that a good pathology report will evaluate and audit the quality of other services such as radiology, surgery, and oncology. The aim of this paper is to outline how this parameter can be audited by surgeons and pathologists to improve both communication and standards.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies in the developed world and with the ‘westernisation’ of diets, other parts of the world are coming to bear a similar burden. Worldwide, CRC ranks third in terms of incidence,, behind lung and breast cancer, but second in terms of mortality.1 Surgery is still the most successful treatment in the vast majority of cases. However, not all surgical specimens have clear resection margins (RM), which is an important parameter in terms of treatment outcome. Advancements in surgery, radiology, oncology, and pathology compel researchers to critically analyse the evidence.2 This paper is designed to review the literature and present the findings on this important topic. To that aim, the longitudinal and circumferential RM (CRM) have been examined.
SPECIMEN PREPARATION3,4
The specimen should preferably be received fresh and intact, i.e., it should not be opened in theatre as this interferes with the assessment of both the CRM and the serosal involvement. The mesorectal and mesocolic planes of the surgical resection were then evaluated and photographed to keep a permanent record of the assessment, and to facilitate discussion at multidisciplinary team meetings (MDT) (Figure 1a and 1b). The non-peritonealised CRM was inked to facilitate histopathological assessment of margin involvement (Figure 1b, 1c, and 1d [blue arrow]). This is not limited to rectal cancers and should include colon cancers with associated circumferential colonic (non-peritonealised) RM, such as caecal and ascending colon tumours. After fixation, when the specimen was left for at least 24–48 hours, the bowel was opened anteriorly above and below the tumour. The neoplasm was then sectioned at 3–4 mm transversely to produce whole (large) mount slices that include the tumour, adjacent lymph nodes, serosa, and CRM. Photography of these slices is recommended to provide a permanent record and to complement the verbal pathology report at the MDT meeting. Blocking the specimen should be according to The Royal College of Pathologists minimum dataset (RCPath MDS), and every effort should be made to retrieve as many lymph nodes as possible (minimum of 12).
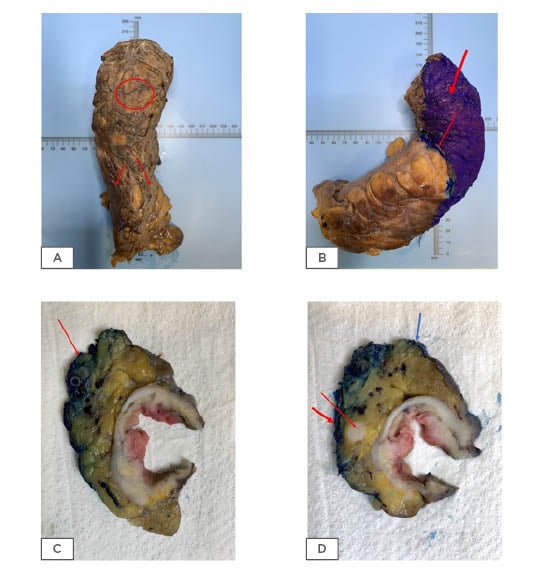
Figure 1: Colorectal resection specimens.
Mesorectal surface with no defects indicating good quality surgery (A: circle, B) which is inked prior to dissection (B). The peritoneal reflection is also shown (A: two arrows and B: thin arrow). Cross section of rectal tumour showing the CRM (C: red arrow and D: thick red arrow) and the peritoneal reflection (D: blue arrow). An involved lymph node near the circumferential resection margin (D: thin red arrow).
LONGITUDINAL RESECTION MARGINS
Traditionally, radical surgery for CRC has included a significant removal of uninvolved parts of the colon. A paper by Miles in 19085 contains a reference to abdominoperineal excision for sigmoid cancer; this practice carried with it an unnecessary compromise of the sphincter, leaving patients with left sided, sigmoid colon with a permanent stoma and the associated morbidity and mortality.
Subsequently, a 5 cm rule was introduced to minimise the huge morbidities associated with removal of long sections of healthy colon. The rule remained until Williams et al.6 challenged the concept and concluded their paper with this statement: “The application of the 5 cm rule of distal excision may cause patients with low rectal cancer to lose their anal sphincter unnecessarilly.”
At a similar time, the St Marks group7 suggested that 2 cm was a safe margin after analysing a cohort of 334 patients who survived radical restorative operations for rectal adenocarcinoma. The length of rectum below the tumour measured on fixed, pinned-out pathologic specimens was ≤2 cm in 55 patients (Group 1), 2–5 cm in 177 (Group 2), and ≥5 cm in 102 (Group 3). The Dukes’ classification, histologic grade, and extent of local tumour spreading was similar in the three groups. Overall crude 5-year survival rates for Groups 1, 2, and 3 were 69.1%, 68.4%, and 69.6%, respectively. Corresponding cancer-specific death rates were 25.5%, 23.2%, and 21.6%. These rates were also similar in matching pathologic subgroups of the three main groups. Of 23 observed or suspected local recurrences, there were 4 recurrences in Group 1 (7.3%), 11 in Group 2 (6.2%), and 8 in Group 3 (7.8%). They suggested that a margin <2 cm below a rectal carcinoma did not affect survival or local recurrence adversely.
Subsequently, Kirwan et al.8 suggested that 1 cm was a safe margin and the follow-up paper from the same group showed the effect of their original work on sphincter saving.9 The proposed safe distal RM became even smaller with the paper by Karanjia et al.,10 when the authors suggested that the reduction of RM provided total mesorectal excision (TME) and properly performed washout, without increasing local recurrence or compromising survival. Twenty years later a published validation of the work by Karanjia et al.10 came from the Cleveland Clinic, entitled ‘Does sub-centimetre distal resection margin adversely influence oncological outcome,’11 as they concluded that <1 cm longitudinal resection margin (LRM) does not influence outcome.
All of the above papers indicate that sphincter preservation surgery is now more achievable than 40 years ago due to an understanding of rectal cancer’s biological behaviour, and also the significant advances in technology. Pathologists audit the closeness of the distal RM and gauge the frequency of anterior resection (AR) versus abdominoperineal resection (APR). It is anticipated there will be more AR than APR in most practices; however, this is a regular clinically audited schedule, because clinical situations like sphincter function play a major role in determining whether the patient is better served with AR or APR.
Longitudinal spread of rectal cancers is very unlikely; thus, it is not essential to assess the longitudinal margins histologically if one of the tumour margins is ≥30 mm. The only piece of information missing from these surgical papers is that the submucosal spread of poorly differentiated CRC can spread >1 cm without being ‘intraoperatively’ noticeable. For this reason, it is recommended that with poorly differentiated adenocarcinoma it is mandatory to examine the resection end, otherwise it is not essential.3 In a recent paper by Lee et al.,12 1,343 primary colonic cancer patients were reviewed and designated LRM to <3 cm, ≥3, and <5 cm. The results showed that with increasing length of LRM, the number of retrieved lymph nodes tend to increase (19.5 ± 12.0, 22.1 ± 12.8, and 30.0 ± 16.2; p<0.001). However, the study showed that the 3-year disease-free survival and 5-year overall survival were not significantly different between the groups.
RECTAL CIRCUMFERENTIAL RESECTION MARGIN
Currently, for patients with mid and lower rectal cancer, TME is regarded as the standard of surgical treatment.13 The pioneering work of Heald et al.14 showed the importance of TME in lowering the risk of regional recurrence by applying sharp dissection along the embryological plane. This approach aimed to produce a specimen with complete mesorectal excision, ensuring completeness of excision without disturbing the ‘holy plane’.15 Subsequently, Quirk et al.16 complemented the work of Heald and colleagues to show that involvement of the CRM is associated with a high percentage rate of local recurrence and low survival. Therefore, it was suggested in a series of papers that positive CRM could be due to one, or any combination of factors: poor standard of surgery, aggressive disease, tumour location, and male sex.
The association between a patient’s outcome and the assessment of the intact mesorectum has been analysed by Nagtegaal et al.,13 using the following grading system:
A (3) (Good). Complete. Mesorectal (MRR):
The mesorectum should be smooth and with a good bulk to the mesorectum both anteriorly and posteriorly. The distal margin should appear adequate with no coning. Any defect should not be >5 mm deep.
B (2) Nearly complete. Intramesorectal (IMR):
There should be a moderate bulk to the mesorectum with minor irregularity of the mesorectal surface with a moderate degree of coning of the specimen distally and the muscularis propria (MPR) should not be visible. There could be moderate irregularity of the CRM.
C (1) (Poor). MPR:
There should be significant defects in the mesorectal tissue with deep cuts into the MPR. The CRM will be very irregular and formed by the MPR in places.
The Nagtegaal et al.13 paper showed conclusively that significant breach of the mesocolon is associated with worse outcomes, as seen in Table 1.

Table 1: Outcomes associated with significant breach of mesocolon.
The group went further by stating that only Grade A was associated with good prognosis.17 The quality of mesorectal excision in the 1,382 patients in a Belgian multidisciplinary improvement project who underwent elective resection for mid or low rectal carcinoma was assessed. The results showed that a 2-Grade score distinguishing MRR from the others (IMR and MPR) was found to predict distant metastasis rate, disease-free survival, and overall survival.
Quirke et al.18 showed that the quality of rectal cancer surgery is an important factor in predicting local recurrence and survival. Results showed that high quality TME surgery reduced the incidence of local recurrence and improved the 5-year survival rate from 48% to 68%. Hence, pathologic assessment of the resection specimen has been shown to be a sensitive indicator to the quality of rectal surgery by grossly assessing the surgical planes of dissection and the CRM as a means of quality control.19
In the UK, the national recommendation suggests that CRM positivity in rectal cancer should be below 15%.20 The pathologist is required to measure the tumour beyond the MPR, recorded in mm, as it relates to prognosis. Extramural extension of the tumour into the mesorectum in rectal cancer of ≥5 mm is associated with worse prognosis. This is specifically pertaining for T3 tumours, which form the majority of rectal cancers. A study performed in Erlangen, Germany,21 demonstrated that the division into pT3a (≤5 mm spread into the mesorectum) and pT3b (>5 mm) carries a risk of locoregional recurrence rates of 10.4% for pT3a and 26.3% for pT3b. The cancer-related 5-year survival rates were 85.4% for pT3a and 54.1% for pT3b. A paper recently published on the importance of accurate measurement of extramural spread in rectal cancer argued that it should be included in any TNM classification as it has a direct influence on outcome.22 This study confirmed that the pT3a has a 30% advantage over pT3b. Subsequently, the European Society for Medical Oncology (ESMO) published guidelines recommending the subclassification of the category T3 using MRI in the assessment of extramural invasion.23 However, despite the data presented, the American Joint Committee on Cancer (AJCC) has not yet included the proposal into TNM version 8.
By convention, the CRM is considered positive if the distance between any tumour cells and the CRM is ≤1 mm.4,24
A positive CRM can be due to a direct tumour extension or a tumour within lymph nodes, veins, lymphatics, or around nerves4 (Figure 1c and 1d).
However, not all types of positive margins carry the same risk for local recurrence. For example, Nagtegaal and Quirke25 showed that a positive CRM due to an involved lymph node (Figure 1d) was associated with a lower risk of local recurrence than a positive CRM due to direct extension (12.4% versus 22.1%, respectively), and no greater risk than that of CRM-negative tumours. Therefore, the type of affected tissue leading to a positive CRM should be specified in the pathology report and its significance discussed in local MDT meetings.4 In the case of a positive CRM based solely on an intra-nodal tumour contained by the lymph node capsule, it is recommended to leave a comment indicating that the risk of recurrence might not differ significantly from that of CRM-negative tumours.
THE NEW CONCEPT OF COMPLETE MESOCOLIC EXCISION
The assessment of non-peritonealised RM in colonic tumours similar to rectal cancers was popularised initially by Bokey et al.26 in their study of patients undergoing a potentially curative, elective colonic resection at Concord Hospital from 1971–1995. By applying the same principle to rectal cancer dissection, the results showed improved survival after adjustment for other known prognostic factors. Subsequently the paper by Hohenberger et al.27 showed that by undertaking CME, the local 5-year recurrence rates in colon cancer reduced from 6.5% in the period from 1978–1984, to 3.6% from 1995–2002. Additionally, the 5-year survival rates increased from 82.1% to 89.1%. Bateman et al.28 later showed that retroperitoneal surgical margin (RSM) tumour involvement occurs within a considerable number of distal caecal and proximal ascending colon carcinomas. The rate of RSM tumour involvement identified here is similar to a previously published local recurrence rate of 10% in caecal carcinoma, suggesting that RSM tumour involvement may be a predictor of recurrence in these tumours. They further suggested that patients with distal caecal or proximal ascending colon carcinoma and RSM tumour involvement may benefit from postoperative radiotherapy.
West et al.29 have produced a series of excellent work in this area and showed that with high vascular ligation, the surgeon achieves larger surface area of mesentery that includes more lymph nodes. The protocol devised is identical to rectal cancer and includes:
- Specimen ideally received fresh.
- Open, but leave the area around the tumour intact.
- Fix for at least 48 hours.
- Photograph anteriorly and posteriorly.
- Serial sectioning through the tumour at 3–5 mm intervals.
- Further photographs of the cross sections.
- The grading is similar to rectal cancer and falls into three categories:
- Mesocolic plane (MCP): Smooth serosal/ mesocolic mesentery or only small minor defects.
- Intramesocolic plane (IMCP): The defect is present but not revealing the MPR.
- Muscularis propria plane (MPP): Major defect(s) in the mesocolon reaching the MPR are present.
The study looked retrospectively at 399 cases in which every specimen followed the above protocol. Results showed 15% overall survival benefit comparing MCP over MPP specially in Stage III.
In a recent study comparing 364 patients in the CME group, with 1,031 patients in the non-CME group, the disease-free survival was significantly higher after CME, with 4-year disease-free survival of 85.8% after CME and 73.4% after non-CME.30
POLYP CANCER (PT1 ADENCARCINOMA) RESECTION MARGIN AND RISK OF RESIDUAL DISEASE
The status of the RM in a malignant colorectal polyp is important in predicting the potential for an adverse outcome.
It is important to specify whether the margin is the deep stromal or the mucosal margin, as more extensive surgery is usually indicated when the stromal margin is involved and further local excision may be necessary if the mucosal margin is involved.20 In the literature, there has been considerable discussion on the degree of margin clearance and what is regarded as acceptable to classify the tumour as completely excised. The agreement is that a clearance of 0 mm and distance of <1 mm is an indication for further surgery due to incomplete excision; however, other investigators would use <2 mm as a cut-off. Currently, the European Guidelines recommend that clearance of ≤1 mm signifies a positive margin.20 In a study of 1,900 patients, residual disease was more frequent in patients with a positive rather than a negative margin (30% versus 3%).31 Margin positivity on its own, in the majority of studies, did not appear to be an independent risk factor for lymph node metastasis, with the risk of lymph node metastasis being similar in patients with and without margin involvement (9.2% versus 7.2%).31 Further treatment may be taken when other high-risk factors are present. If there is uncertainty about margin involvement in cases with no other high-risk features, endoscopic follow-up looking for local recurrence is reasonable and is considered good practice. The ACPGBI position statement stratified the risk factors for malignant polyps and the authors advise following this recommendation (Table 2).32 An accurate measurement of the tumour to the closest deep margin should be recorded. However, diathermy artefact creates a false plane which can hamper assessment of the distance between the tumour cells and the RM. The zone of this artefact could be several millimetres and this measurement of clearance should not be present in the inner aspect of the diathermy zone. However, any infiltration by malignant glands into the diathermy zone is regarded as margin involvement (0 mm; R1 status), as it is not possible to confidently determine the true extent of infiltration in this situation.33
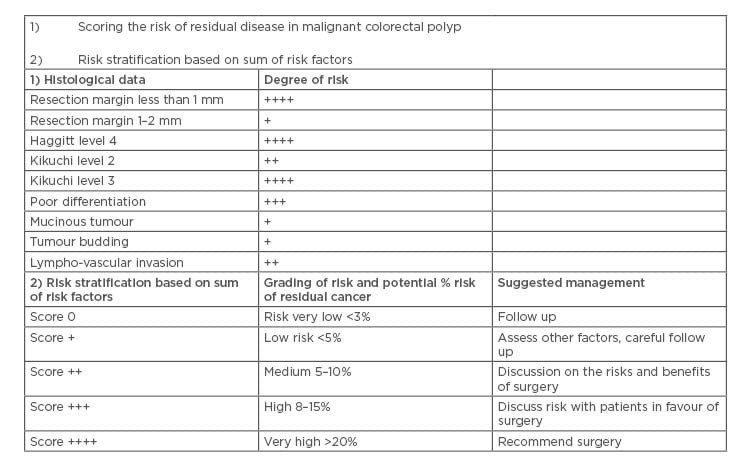
Table 2: Risk factors for residual disease and suggested management plans in a malignant colorectal polyp.
Adapted from Williams et al.32
CONCLUSION
Pathologists play a key role in the modern multidisciplinary management of patients with CRC. Pathological assessment of the resected specimen not only provides key prognostic information, but also allows evaluation of the quality of the surgery, accuracy of radiology, and an assessment of response to neo-adjuvant therapy. A useful component in facilitating feedback on the quality of surgical specimens is keeping a permanent record of each specimen using digital photography. These images should be stored in a departmental archive and should be actively used in MDT meetings for feedback to clinical colleagues. In addition, they can be used for education, research, and audit purposes.19 In essence, the pathologist assesses the quality of surgery and the surgeon assesses the quality of the pathologist’s report.








