Speakers: Brian E. Lacy,1 Morris Gordon,2 Lukas Michaja Balsiger,3 Sachin Srinivasan,4,5 Sundeep Lakhtakia,6 Alvin J. Freeman,7 Cesare Hassan,8 Henry P. Parkman9
1. Division of Gastroenterology and Hepatology, Mayo Clinic, Jacksonville, Florida, USA
2. University of Central Lancashire, Preston, UK
3. Translational Research Center for Gastrointestinal Disorders, University of Leuven, Belgium
4. University of Kansas School of Medicine, Kansas City, USA
5. Kansas City VA Medical Center, Kansas City, Missouri, USA
6. Asian Institute of Gastroenterology, Hyderabad, India
7. Emory University School of Medicine, Atlanta, Georgia, USA
8. Humanitas University, Milan, Italy
9. Temple University School of Medicine, Philadelphia, Pennsylvania, USA
Disclosure: Lacy has served on scientific advisory boards for Allakos, Allergan, Arena Pharmaceuticals, Cosmos, Ironwood, and Salix Pharmaceuticals; and as a consultant for Viver Health. Gordon has received no funds from any pharmaceutical or formula company in any form for travel or other related activities since 2019. Freeman has served as a consultant for AbbVie and Takeda; and received research support from the National Institutes of Health (NIH), CFF, Travera Therapeutics, and Allergan. Hassan has received consulting or research fees and/or worked on an advisory board for Alphasigma, Ipsen, and Norgine. Parkman has received consulting fees from Evoke Pharma, AEON, and Takeda Pharmaceutical Company; research grants from Medtronic and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); and has participated in clinical trials with Vanda Pharmaceuticals Inc. and Neurogastrix. Balsiger, Srinivasan, and Lakhtakia have declared no conflicts of interest.
Acknowledgements: Writing assistance was provided by Nicola Humphry, Nottingham, UK.
Support: The publication of this article was funded by Abbott; its Global Medical Affairs department selected the abstracts for inclusion.
Citation: EMJ Gastroenterol. 2022;11[4]:4-14. DOI/10.33590/emjgastroenterol/10070679. https://doi.org/10.33590/emjgastroenterol/10070679.
Meeting SummaryDigestive Disease Week (DDW) 2022 was held both in San Diego, California, USA, and virtually. The meeting featured cutting-edge research and education in the field of gastroenterology, of which eight treatment-related presentations are summarised in this article. Two presentations covered recent patient surveys. The first highlighted that many patients with chronic idiopathic constipation (CIC) remain unsatisfied with the control of their symptoms, while the second highlighted that most patients with gastro-oesophageal reflux disease (GORD) experience reflux symptoms despite proton pump inhibitor (PPI) therapy.
A survey of gastroenterologists showed that there was a highly varied approach to irritable bowel syndrome (IBS) with predominant diarrhoea (IBS-D) in Europe. Morris Gordon from the University of Central Lancashire, Preston, UK, presented data from a systematic literature review, which found that there was currently insufficient evidence to confirm whether probiotics might be an effective treatment for paediatric functional constipation. On a more positive note, the results of a pilot study combining pantoprazole and itopride in the treatment of GORD suggested that this combination is promising, and an observational study indicated that the use of pancreatic enzyme supplements in pancreatic-sufficient children with acute recurrent pancreatitis may decrease pancreatitis episodes.
To bring gastroenterologists up to date, Cesare Hassan from the Humanitas University, Milan, Italy, explained why it is important to optimise colonoscopy bowel preparation, with some key approaches to achieve this, and Henry P. Parkman from Temple University School of Medicine, Philadelphia, Pennsylvania, USA, reviewed the current and future approach to medical therapy in patients with gastroparesis.
Chronic Idiopathic Constipation and Treatment Satisfaction: Prescription Versus Over-the-Counter Medications
Brian E. Lacy
Brian E. Lacy from the Division of Gastroenterology and Hepatology at the Mayo Clinic, Jacksonville, Florida, USA, presented the results from two observational, cross-sectional, online surveys of adults in the USA, which aimed to gather information on the patient perspective of treatments for CIC.1 The first survey used random stratified sampling to ensure a demographic composition representative of the USA, and the second was completed by participants of the first survey who self-reported having IBS, constipation, or diarrhoea.
Both surveys were available for approximately 1 week each month between August 2020 and August 2021. Participants with CIC were identified using Rome IV criteria, including experiencing at least two out of six predefined constipation symptoms for over 6 months, and failure to qualify for constipation-predominant IBS, indicated by the absence of recurrent abdominal pain. Across 24,089 participants, 8.7% (n=2105) met the Rome IV criteria for CIC, of which 28.9% (n=608) reported currently taking prescription medication, and 45.1% (n=949) reported currently taking an over-the counter (OTC) medication and no prescription medications.
Among those participants with CIC who were taking medication, the prescription medication cohort had a lower proportion of females (60.7% versus 73.0%; p<0.001), a younger mean age (41.9 versus 48.3 years; p<0.001), a slightly lower body mass index (BMI: 27.2 versus 28.0; p=0.017), and higher proportions of participants that were educated beyond high school and employed (79.6 versus 75.1; p=0.041 and 64.9% versus 49.1%; p>0.001, respectively) compared with the OTC cohort.
The most reported symptom leading to medication use in the prescription medication cohort was abdominal pain (79.9%), whereas in the OTC medication group it was abdominal discomfort (62.5%). Of the participants who were taking OTC medication, roughly half (55.9%) had not requested prescribed medication because they felt that the OTC medications worked well. Others had not asked for prescriptions because they preferred not to take a prescribed medication (24.6%), they were not aware of any prescribed medications that would help them (21.6%), or they were concerned about the cost (21.2%). The most reported prescription medication currently taken was linaclotide (29.1%), followed by citalopram (22.9%), lactulose (18.4%), lubiprostone (14.6%), and dicyclomine (13.8%).
Overall, participants currently taking prescribed medication reported higher satisfaction with control of bowel symptoms (49.3% versus 27.2%; p<0.001) and abdominal symptoms (48.8% versus 29.8%; p<0.001) compared with those on OTC medications. Over half of the participants taking linaclotide reported that they were quite or very satisfied with their bowel and abdominal symptoms (57.6% and 56.5%, respectively).
Lacy concluded that there remains a considerable disease burden in CIC, and many patients remain unsatisfied with the control of their symptoms. Those currently taking prescription medications are generally more satisfied than those currently taking OTC medications.
Probiotics for Treatment of Functional Constipation in Children: A Cochrane Systematic Review
Morris Gordon
Functional constipation (chronic constipation with no identifiable underlying cause) accounts for up to 25% of visits to paediatric gastroenterologists.2 Gordon described the results of a systematic review of the literature to evaluate the efficacy and safety of probiotics for the management of functional constipation in children.3,4
Fourteen studies (n=1,127 randomised participants) were included, of which 12 assessed probiotics and two investigated synbiotic preparations. All studies were two-armed, with participants in the control arm receiving placebo, osmotic laxatives, magnesium oxide, or paraffin.
Of the studies that compared probiotics with placebo, four reported efficacy, with pooled estimates suggesting that there may be little difference in treatment success (relative risk [RR]: 1.29; 95% confidence interval [CI]: 0.73–2.26; low-certainty evidence). Five reported the number of withdrawals due to adverse events, again indicating that there may be no difference (RR: 0.64; 95% CI: 0.21–1.95; low-certainty evidence).
Several studies evaluated probiotics as an adjuvant therapy for osmotic laxatives. Pooled estimates from three studies suggested that probiotics may not improve the frequency in defecation (mean difference: ‑0.01; 95% CI: -0.57–0.56; low-certainly evidence). Two studies reported efficacy, with results indicating that there may be no benefit in adding probiotics to osmotic laxatives (RR: 0.95; 95% CI: 0.79–1.15; low-certainty evidence). In terms of withdrawals due to adverse events, it was unclear from the pooled estimate from the three studies whether there was any difference between the treatment groups (RR: 2.86; 95% CI: 0.12–68.35; very low-certainty evidence).
Although the pooled data suggest that there may not be a difference in efficacy or safety between the use of probiotics and either placebo or other interventions, evidence was of low- to very-low-certainty. In the full report of this systematic review, Wallace et al.4 explained that the available evidence is incomplete in several ways. Firstly, few studies differentiated between treatment-naïve children and those who had had years of failed interventions; furthermore, the primary studies did not evaluate the severity and type of constipation. Secondly, the variety of probiotics and synbiotics used in the studies mean that the pooled evidence can only consider the broad class of these interventions. Finally, most studies had a short follow-up time, yet functional constipation is a chronic condition.
Gordon concluded that current evidence is not yet sufficient to confirm the efficacy of probiotics in children with functional constipation, and that further research is needed.
Case-Based Evaluation Shows Highly Varied Approach to Predominant Diarrhoea Irritable Bowel Syndrome Treatment by European Experts
Lukas Michaja Balsiger
Lukas Michaja Balsiger from the Translational Research Center for Gastrointestinal Disorders, University of Leuven, Belgium, presented data from a case-based online survey of 24 European experts, which was designed to assess management approaches to IBS-D.5 Experts were presented with 10 clinical vignettes, each varying in symptom predominance, patient presentation, demographics, and psychosocial factors.
For the diagnosis of IBS-D in a simple case, without modifying factors, the majority of experts would order a complete blood count and/or coeliac serology (96%), and tests for stool calprotectin (91%) and thyroid stimulating hormone (78%). Nine percent of experts would order an endoscopy. If the patient presented with risk factors for bile acid diarrhoea (e.g., cholecystectomy), 35% of experts would test for bile acid malabsorption and, if the patient presented with risk factors for chronic pancreatitis (e.g., alcohol abuse), 48% of experts would perform abdominal ultrasound. In patients whose symptoms coincided with their travel history, 74% of experts would analyse the stool for parasites, and 48% would order a stool culture and/or PCR test to exclude an infection.
The approach to treatment varied widely depending on the individual predominating symptom and the presence of modifying factors (Figure 1). The most frequently chosen treatment options for a simple case of IBS-D were dietary intervention at first-line (40% of experts), antibiotics at second-line (26%), and ondansetron at third-line. For IBS-D with post-infectious onset, 30% of experts would prescribe antibiotics at first-line. For patients with risk factors for bile acid diarrhoea, experts would use bile acid sequestrants at first- (48%) or second-line (35%). In cases where pain was the predominant symptom, the highest consensus for treatment was the use of antispasmodics at first-line (52%), with 30% and 40% of experts choosing to use neuromodulators at second- and third-line, respectively.
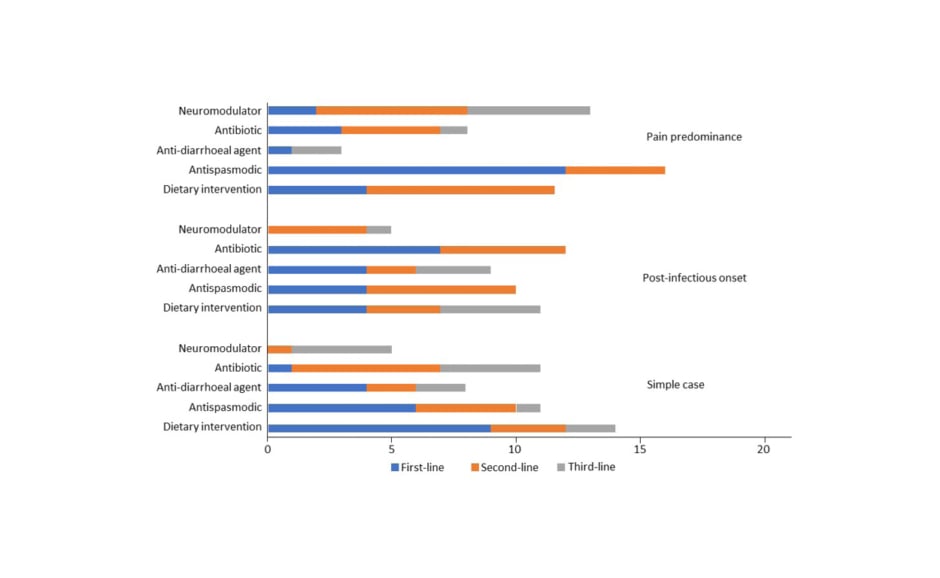
Figure 1: Number of experts that chose each treatment option in three types of diarrhoea predominant irritable bowel syndrome at first-, second-, and third-line.5
Balsiger emphasised that experts often chose a combined approach over monotherapy in each line of therapy, highlighting the complexity of treating IBS-D.
Symptom Profile, Proton Pump Inhibitor Therapy, and Diagnostic Testing in Patients with Refractory Gastro-oesophageal Reflux Disease: A Population-Based Study
Sachin Srinivasan
Up to 40% of patients with GORD have a partial or complete lack of response to the standard dose of proton pump inhibitor drugs.6 Sachin Srinivasan from the University of Kansas School of Medicine, Kansas City, USA, and Kansas City VA Medical Center, Kansas City, Missouri, USA, explained that current recommendations for the treatment of refractory GORD are based primarily on expert opinion, and there is a scarcity of patient-reported perspectives and outcomes.7
To collect patient-reported data regarding the diagnosis of GORD or refractory GORD, symptoms, treatment, and testing, a population-based survey was conducted using an online platform followed by an email inviting individuals to participate in the survey. Of 283 participants who completed the survey, 197 reported being formally diagnosed with GORD or refractory GORD within the past 3 years (36%), 3–10 years (32%), or over 10 years ago (31%). Among these 197 participants, 23% were currently managed by their primary care provider, 32% by a gastroenterologist, and 9% were managing their own symptoms.
Most (74%) participants with GORD were using a PPI: 58% on a daily basis, 26% on a twice-daily basis, and 9% less than once daily. Continuing troublesome reflux symptoms were reported by 72% of PPI users, and the majority of PPI users expressed concerns regarding long-term PPI usage. Compared with participants with GORD, those with refractory GORD were significantly more likely to report undergoing upper gastrointestinal endoscopy (93% versus 85%, respectively; p=0.02) and manometry (27% versus 13%, respectively; p<0.01).
Srinivasan concluded that while most participants with GORD were receiving PPI therapy, nearly three-quarters continued to experience troublesome reflux symptoms. In addition, the survey revealed considerable variability in the diagnostic tests performed in patients with persistent GORD symptoms.
Fixed-Dose Combination of Pantoprazole and Itopride in Patients with Refractory Gastro-oesophageal Reflex Disease and Dyspepsia: A Pilot Study
Sundeep Lakhtakia
One potential approach to improving the success of GORD treatment is combining a PPI with a prokinetic agent; several studies have reported that this combination may be more effective than PPI alone.8-10 Sundeep Lakhtakia from the Asian Institute of Gastroenterology, Hyderabad, India, described the results of a pilot study designed to evaluate the efficacy and safety of fixed-dose combination therapy with pantoprazole (a PPI) and itopride (a prokinetic) in refractory GORD patients with overlapping dyspepsia symptoms.11
Participants with at least a 3-month history of pantoprazole-refractory GORD and dyspepsia (n=50) were administered pantoprazole 40 mg and itopride 150 mg once daily for 6 weeks. Participants were asked to complete the GORD Symptom Assessment Scale (GSAS) questionnaire12,13 at baseline and at Week 6, and adverse events were monitored throughout treatment. The majority (84%) of participants were male and the mean age was 44 years (range: 21–69 years). During the study, five patients were lost to follow-up and four patients dropped out because they did not achieve symptomatic relief, one of whom also experienced an adverse event.
The most frequently reported symptoms at baseline were heartburn (84%) and bloating (84% [Figure 2]). Heartburn was also the most commonly reported symptom at Week 6, but at a significantly lower frequency (26.8%; p<0.001). All symptoms were significantly less frequently reported (p<0.01) at Week 6 compared with baseline. Three adverse events were experienced by two patients (4.3%) at Week 4, all of which were resolved.
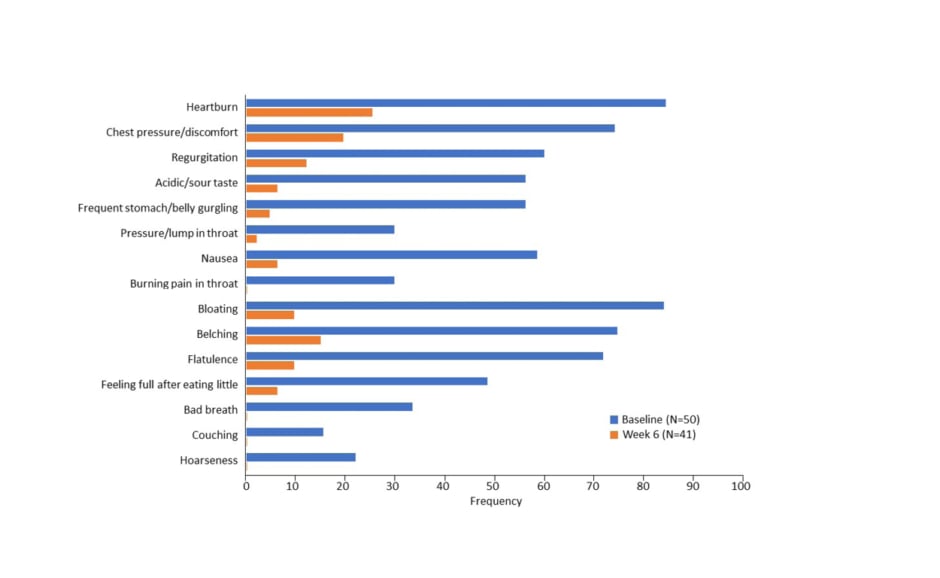
Figure 2: Frequency of Gastro-oesophageal reflux disease Symptom Assessment Scale (GSAS) symptoms from baseline to Week 6.8
Lakhtakia concluded that fixed-dose treatment with pantoprazole plus itopride had a favourable safety and efficacy profile in patients with pantoprazole-refractory GORD and dyspepsia symptoms in this pilot study, and that this combination warrants further investigation in a larger study.
Pancreatic Enzyme Supplement Use in Children with Acute Recurrent Pancreatitis (PAUSE): An Observational Study
Alvin J. Freeman
Despite the increasing incidence and awareness of acute pancreatitis and acute recurrent pancreatitis in children over the past 10–20 years,14-19 paediatric-specific management guidelines are lacking.20
Alvin J. Freeman from Emory University School of Medicine, Atlanta, Georgia, USA, presented observational data from the PAUSE study, which aimed to assess the use of pancreatic enzyme (PE) supplementation in pancreatic-sufficient children with acute recurrent pancreatitis.21 Participants were identified retrospectively from the INSPPIRE-2 (NCT03672422)22,23 cohort, a large multicentre prospective cohort study.
Of 432 pancreatic-sufficient children with acute recurrent pancreatitis, 54 (12.5%) were treated with PE. During treatment, 40.7% of participants experienced ≥1 acute pancreatitis episode (median episodes: 1 [range: 1–4]), and 59.3% reported no further pancreatitis attacks. These two groups of patients were similar in age, gender, age at enzyme initiation, and lifelong number of acute pancreatitis episodes, as well as follow-up times.
Of those children who continued to experience acute pancreatitis episodes after starting PE therapy, the median number of episodes was statistically and significantly less after starting PE therapy than before starting PE therapy (1 [range: 1–4] versus 2 [range: 1–6], respectively; p=0.04).
Freeman concluded that this is the first study to suggest that the use of PE in pancreatic-sufficient children with acute recurrent pancreatitis may decrease the number of pancreatitis episodes experienced. However, he stressed that placebo-controlled clinical trials will be needed to confirm these findings.
How Do I Optimise Colonoscopy Bowel Preparation and Measure Performance Improvement When Needed?
Cesare Hassan
Hassan emphasised the importance of optimising bowel preparation for colonoscopy using three key points: gastroenterologists need to avoid futile repeat colonoscopy in patients that fail their first bowel preparation; evidence-based medicine can only guide the first attempt at bowel preparation; and clinical trials report a level of efficacy for bowel preparation that does not seem to be achieved in primary or secondary care.24
Inadequate bowel preparation has been shown to result in a 3-fold increase in the risk of missed adenomas during colonoscopy.25 In a prospective observational study based on the Boston Bowel Prep Scale (BBPS; 0–9), the rate at which adenomas >5 mm were missed was 5.6% at BBPS=3 and 15.9% at BBPS=1.25
Rather than being intended to clean the bowel, Hassan emphasised the need to convince patients that bowel preparation is intended to maximise detection of abnormalities. Overall, 26% of patients consider bowel preparation to be the most burdensome aspect of colonoscopy, and this represents a major barrier for its acceptance.26,27
The Secretary General of the European Society of Gastrointestinal Endoscopy, Raf Bisschops, recently published advice for bowel preparation,28 and these recommendations follow the approaches to optimisation presented by Hassan.
A 1-Day Low-Fibre Diet is Sufficient
There are two main options for a bowel preparation diet: a low residue (fibre) diet and a clear liquid diet. Both diets are similarly effective, but the former was found to be significantly more tolerable to patients.29 In addition, a Spanish study has shown that there is no significant difference between a 1-day bowel preparation diet and a 3-day bowel preparation diet in terms of adequacy of bowel cleansing; a global BBPS ≥2 was achieved in 82.7% and 85.6% of patients, respectively.30
Split-Dose Regimens Are Superior to Same-Day Dosing Regimens
Taking the bowel preparation solution in two doses has been shown to be more effective at bowel cleansing.31 For example, taking 2 L of polyethylene glycol (PEG) as a split dose, rather than a single dose the day before colonoscopy increased the adenoma detection rate by a RR of 1.22 in a subjects undergoing their first colonoscopy (n=690).31 Splitting the dose improves terms of bowel preparation compared with taking a single dose, independent of the laxative or volume used.32
However, in addition to the efficacy of split-dose regimens, more patients report impairment to working performance with split-dose regimens compared with same-day regimens (odds ratio: 4.04).33
A Low Volume Is Generally Sufficient
Hassan explained that the efficacy of split-dose regimens has essentially marginalised the question of bowel preparation solution volume. A meta-analysis of the efficacy of low-volume versus high-volume split-dose PEG bowel preparation regimens found no significant differences between the two regimens.34 Even a very low dose of 1 L PEG (plus ascorbate) was found to be non-inferior to a 4 L dose of PEG in a randomised trial in Italy (NCT03742232).35,36 However, Hassan emphasised that bowel preparation volumes should be modified for patients with severe constipation or those who are otherwise at risk of inadequate bowel preparation.
Encourage Patients to Follow a Split-Dose Regimen
Although gastroenterologists recommend using a split-dose regimen for bowel preparation, Hassan explained that many patients prefer a single-dose regimen.37 The female sex and a colonoscopy appointment time before 10:00 a.m. were associated with reduced uptake of the split-dose regimen (odds ratio: 0.74 and 0.14, respectively).37 However, it is possible that increased encouragement to follow a split-dose regimen, via a dedicated leaflet, may help to improve the adherence of subjects to this bowel preparation regimen.38
Use Standardised Scoring Systems to Record Bowel Preparation Results
Quality indicators proposed for bowel preparation include the frequency with which the procedure note documents the quality of preparation, and the frequency with which bowel prep is adequate to allow the use of recommended surveillance or screening intervals.39 The quality of the bowel preparation can be scored using one of several validated scales, including the BBPS. However, Hassan noted that, in the future, gastroenterologists are likely to make increasing use of artificial intelligence scoring programs, such as that the ENDOANGEL system recently developed in China.40
Hassan summarised the key takeaways for optimisation of bowel preparations as follows: prescribe a low-volume of an approved bowel preparation solution; encourage the use of a split-dose regimen; advise patients to follow a low-fibre diet for one day prior to colonoscopy; adapt bowel preparations for challenging patients; and target an ‘excellent’, rather than ‘adequate’ level of bowel cleansing.
Medical Therapy in Gastroparesis: Diet, Prokinetics, Anti-emetics, Neuromodulators, and Botulinum Toxin
Henry P. Parkman
Parkman emphasised the chronic nature of gastroparesis and the continuing high disease burden, citing an analysis of registry data from the USA,41 which showed that just 28% of patients experienced a reduction in Gastroparesis Cardinal Symptom Index (GCSI) after 48 weeks of standard of care (n=262).42
Patients with Diabetes Should Lower Their Blood Sugar to Reduce Their Symptoms
A study from the USA investigated the use of continuous glucose monitoring combined with insulin pump therapy in patients with gastroparesis and poorly controlled diabetes (HbA1c: >8%; n=45).43 Forty-two participants completed 24 weeks of treatment. The study found that HbA1c levels decreased by 1.1% from baseline to Week 24 (p=0.0002), and this was associated with a statistically significant (p<0.001) improvement in gastroparesis symptoms (change in GSCI score: -6.6). Improvements were observed in symptoms of nausea/vomiting, fullness/early satiety, and bloating/distension, and participants’ liquid nutrient tolerance was also increased.
Patients Should Be Advised to Follow a Low-Fat, Low-Roughage Diet with Small Meals and an Emphasis on Liquid Nutrients
In a small study, patients with gastroparesis (n=12) received a daily high-fat solid meal, high-fat liquid meal, low-fat solid meal, or low-fat liquid meal on four separate days in a randomised order.44 The high-fat solid meal resulted in the greatest increase in total symptom score and nausea severity, whereas the low-fat liquid meal had the least effect on total symptom score and nausea severity. Parkman felt that these data may help to encourage patients to convert to low-fat, liquid-based meals if they are experiencing troubling gastroparesis symptoms.
Anti-emetic Agents to Reduce Nausea and/or Vomiting
Parkman stressed the importance of anti-emetic medication in patients with gastroparesis, explaining that nausea/vomiting were the symptoms generally responsible for reducing the quality of life in these patients.
Delivery of the 5-hydroxytryptamine receptor antagonist, granisetron, via a transdermal patch has been shown to reduce nausea/vomiting in 50% of patients with gastroparesis who were experiencing symptoms refractory to conventional-treatment (n=35).45 A daily dose of the neurokinin 1 receptor antagonist, aprepitant, has also been shown to significantly reduce nausea symptom severity, vomiting, and overall symptoms (by GCSI score) compared with placebo; although it had no effect nausea measured by the Visual Analogue Scale (VAS).46
Prokinetic Agents to Speed Gastric Emptying
Oral or parenteral formulations of the prokinetic agent metoclopramide were approved for use in adults with diabetic gastroparesis over 40 years ago by the U.S. Food and Drug Administration (FDA).46 A nasal spray formulation of metoclopramide, which bypasses the gastrointestinal system, was recently approved for the same indication,46,47 and Parkman felt that this could be a promising formulation for patients with gastroparesis-associated nausea/vomiting.
Due to the lack of approved prokinetic agents for gastroparesis, Parkman explained that gastroenterologists use several drugs off-label to manage gastroparesis symptoms. In clinical practice, Parkman commonly administers erythromycin with a “3 weeks on, 1 week off” pattern, to avoid the tachyphylaxis phenomenon observed by Dhir and Richter.48 He also uses domperidone, which appears to improve symptoms in 60% of patients with gastroparesis, but can produce adverse effects that result in treatment discontinuation in 12% of patients.49,50
Prucalopride, a relatively recent prokinetic agent approved for chronic constipation, has also been evaluated in gastroparesis. In one study (NCT02510976),51 prucalopride 2 mg daily for four weeks improved both gastric emptying and symptoms of gastroparesis in a cohort of 34 patients with predominantly idiopathic gastroparesis.52 In another study (NCT02031081),53 prucalopride 4 mg daily for 4 weeks increased gastric emptying and bowel movement frequency, but did not affect gastroparesis symptoms, in a cohort of 15 patients with predominantly diabetic gastroparesis.54 Parkman suggested that gastroenterologists consider the use of prucalopride in patients with gastroparesis with constipation.
The Potential of Neuromodulators
Central neuromodulators (e.g., antidepressants and antipsychotics) are increasingly employed to treat functional gastrointestinal disorders,55,56 and Parkman highlighted research into the potential use of these agents for gastroparesis.
Parkman explained that although tricyclic antidepressants have been used in clinical practice to treat nausea, vomiting, and abdominal pain associated with gastroparesis, there are limited high-quality data to support this approach. A 14-week, randomised trial (NCT00765895)57 of the tricyclic antidepressant nortriptyline showed no improvement in overall gastroparesis symptoms in patients with idiopathic gastroparesis compared with placebo, although improvements in abdominal pain were observed (p=0.04).58 In an open-label trial, treatment with the atypical antidepressant mirtazapine 15 mg each night for 14 weeks in patients with refractory gastroparesis improved nausea and vomiting at Week 2 and Week 4, yet adverse effects led to treatment discontinuation in 20% of patients.59
Injection of botulinum toxin into the pylorus may improve gastric emptying in patients with gastroparesis, but whether this results in reduced total symptom scores remains unclear.60,61 One study found that patients with low pyloric distensibility were more likely to respond to intra-pyloric botulinum toxin treatment than patients with normal distensibility.62 Parkman felt that in select patients, botulinum toxin injection may be useful to temporarily alleviate symptoms of gastroparesis while further treatments are considered. However, he stressed that repeated injections should be avoided as they could result in scarring of the pylorus.
Parkman summarised his clinical practice approach to the management of patients with gastroparesis: a medication review to identify potentially causative agents; glucose control for patients with diabetes; diet modification to reduce fat and roughage; anti-emetics, as needed; daily prokinetics; neuromodulators, if necessary; and the temporary use of botulinum toxin injections in treatment-refractory patients.








