Abstract
The authors report a case of synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in a patient with Crohn’s disease (CD). SAPHO syndrome is a rare disease characterised by the association, even if not simultaneous, of joint and skin manifestations. A young patient with CD was admitted to the authors’ hospital for the onset of bloody diarrhoea, persistent chest pain, headache, and fever while on maintenance therapy with vedolizumab. At visit, sternocostoclavicular and temporomandibular joints were tender and painful. Magnetic resonance was performed, and showed bone oedema of involved joints, while ileocolonoscopy revealed ulcers in the transverse colon. At laboratory, tests marked phlogosis and Campylobacter jejuni infection was observed. A challenge in differential diagnosis arose: atypical drug-induced extraintestinal manifestations, reactive arthritis, or extraintestinal manifestation directly associated with intestinal flare? In relation to the patient’s age, the involved joints, and magnetic resonance findings, SAPHO syndrome was diagnosed. Systemic steroids were used with a rapid clinical improvement; vedolizumab was withdrawn and ustekinumab was started with sustained clinical response.
Key Points
1. The present case report describes the occurrence of an acute osteochondritis in a young male affected by Crohn’s disease (CD) that the authors diagnosed as synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. This challenging clinical picture raised interest on the problem of differential diagnosis in those patients in which the same presentation can hide several, and even rare, clinical entities.2. This case highlights the great importance of joint management between gastroenterologists and rheumatologists, to optimise clinical outcomes and therapeutic approaches.
3. In this case, ustekinumab allowed control of both intestinal manifestations and sternoclavicular osteitis, proving to be an effective therapeutic approach in patients with Crohn’s disease and SAPHO syndrome.
INTRODUCTION
Inflammatory bowel diseases (IBD) are chronic diseases characterised by an unpredictable course that can be complicated by extraintestinal manifestations (EIM). EIMs affect approximately 50% of patients with IBD at any time during the disease course, and most commonly involve musculoskeletal (causing arthralgia due to arthritis, either axial or peripheral), cutaneous, and ocular systems.1-3 Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome has been considered for a long time a rare form of seronegative spondyloarthropathy. The association between SAPHO and IBD has been known since 1992;4,5 however, it is often underdiagnosed because of its rarity, and similarity with other more common IBD-associated EIMs.
The authors report a case of SAPHO syndrome in Crohn’s disease (CD). The aim is to highlight the challenges in differential diagnosis of EIMs in the context of IBD; and to point out the importance of joint management between gastroenterologists and rheumatologists for a correct diagnosis, an effective therapeutic approach to achieve an optimal outcome, and improved quality of life in patients with IBD.
CASE REPORT
In August 2022, a 24-year-old White male affected by ileocolonic CD was hospitalised in the authors’ IBD unit for the appearance of persistent chest and temporomandibular pain, with remitting fever up to 39 °C.
The patient was diagnosed with CD in 2018 (A2, L3, B1, according to Montreal classification), and regularly followed up in the authors’ outpatient IBD clinic. An early treatment with adalimumab was started at diagnosis due to the presence of multiple unfavourable prognostic factors (extensive colonic involvement, severe endoscopic lesions, young age, anaemia, and inflammatory systemic impact). However, after experiencing primary failure to adalimumab, and subsequently infusion reaction to infliximab, a swap to vedolizumab (300 mg at 0, 4, and 12 weeks, and subsequently every 8 weeks) was initiated in February 2021. Sustained steroid-free clinical remission and endoscopic improvement were achieved after 18 months of vedolizumab treatment. It must be specified that no EIMs were present at diagnosis.
At the last outpatient visit, the patient was in intestinal clinical remission, but complained of dull pain, progressively increasing, and exacerbated by exertion, located in the middle of the chest, and radiating to the right shoulder and neck. The physical examination revealed a warm and erythematous swelling of approximately 4 cm in diameter in the left sternoclavicular joint region, fluctuating, and tender to palpation. Ultrasonography of superficial lymph nodes was negative. In suspicion of synovitis, the initial treatment proposed was ibuprofen 1,200 mg/day, with slight improvement in chest pain, but worsening of headache and temporomandibular joint paint. Furthermore, due to the appearance of a fever up to 39 °C and bloody diarrhoea (4–6 bowel movements/day), the patient was admitted to hospital.
At admission, laboratory tests revealed elevated C-reactive protein levels (26.2 mg/dL) and faecal calprotectin (1,865 mg/kg), together with inflammatory anaemia (haemoglobin: 10.4 g/dL; ferritin: 198 ng/mL). Autoimmunity panel showed slight positivity for c-ANCA (1:40) and anti-PR3 (94 chemiluminescent units; normal value <20 chemiluminescent units), and absence of the allele HLA-B27. Blood and urinary culture, urethral swab for sexually transmitted disease, cytomegalovirus, Epstein–Barr virus, HIV serology, and interferon gamma release assay test were performed with negative results, whereas stool pathogens test revealed a positivity for Campylobacter jejuni. Ileocolonoscopy showed severe activity with ulcers in the transverse colon (Figure 1), in absence of ileal lesions, and histologic examination confirmed colic moderate-severe activity with neutrophilic infiltrate. T1 and T2-weighted fat-suppressed gadolinium-enhanced MRI of temporomandibular and chest joints showed alteration of signal characterised by hyperintensity in the long tandem repeat sequences of the distal epiphysis, and the third distal of the diaphysis of the right clavicle. Areas with similar appearance were found in the proximal and distal portion of the sternal body. After administration of gadolinium, impregnation of the areas described was detected, and the finding was to be referred to areas of bone oedema (Figure 2).
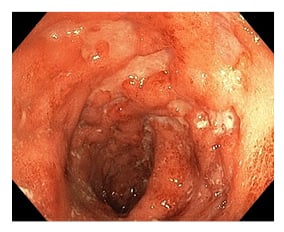
Figure 1: Colonoscopy imaging showing ulcers of transverse colon.
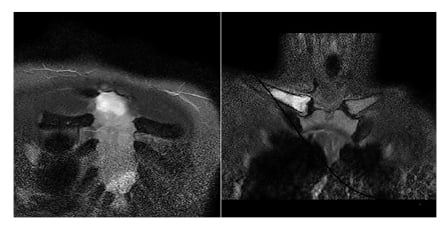
Figure 2: MRI at onset of symptoms, showing hyperintensity in the long tandem repeat sequences of the right clavicle and in the sternal body.
In relation to the patient’s age, the insidious onset initially, with the involvement of the costosternal, and then of the temporomandibular joint, and the presence of fever and MRI findings, SAPHO syndrome was suspected. Since initial treatment with non-steroidal anti-inflammatory drugs was ineffective on articular manifestations, and no improvement was observed after a course of azithromycin 500 mg once daily given for Campylobacter infection, systemic steroids (methylprednisolone 0.75 mg/kg) were started, leading to rapid improvements of joints pain, resolution of fever, and intestinal clinical remission. In light of severe endoscopic lesions, vedolizumab was withdrawn, and the patient was swapped to ustekinumab.
After 1 year of ustekinumab therapy, the patient was still in complete clinical remission for both gastrointestinal and extraintestinal manifestations. MRI of chest joints showed complete resolution of bone oedema and other inflammatory findings (Figure 3). Faecal calprotectin after 1 year was 259 mg/kg.
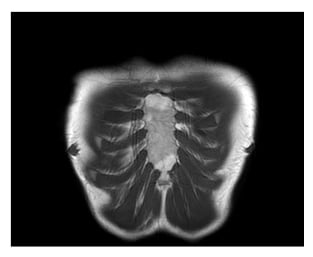
Figure 3: MRI after 10 months, showing resolution of inflammatory findings.
DISCUSSION
The present case report describes the occurrence of an acute osteocondritis in a young male affected by CD, which the authors diagnosed as SAPHO syndrome. This challenging clinical picture raised the interest on the problem of differential diagnosis. In this patient, the authors could, in fact, formulate three main diagnostic hypotheses: atypical drug-induced extraintestinal manifestations, reactive arthritis, or EIMs directly associated with intestinal flare.
The first option was excluded because there is no agreement in the literature regarding the role of vedolizumab in triggering de novo extraintestinal manifestations.6,7 It is theoretically possible that its gastrointestinal selectivity, useful to reduce systemic side effects and adverse reactions, may lead α4β7-expressing lymphocytes to other organs, predisposing patients to develop EIMs with a parallel course to IBD. Some studies, as post hoc analyses of GEMINI-2,8 have shown that the onset or worsening of joint manifestations is less likely with vedolizumab compared to placebo. Other studies have reported the drug as a cause of arthritis, with patients being 28% more likely to develop such EIMs than those treated with anti-TNF-α antagonists.9 In the treatment of those extraintestinal manifestations, both stopping vedolizumab and adding a new drug (e.g. systemic steroids, azathioprine, methotrexate) appear to be effective, and even better results have been reported in patients who switched to ustekinumab.10
As far as concerns the second hypothesis of reactive arthritis, the authors performed an extensive screening for infectious disease, and only a concomitant enteric infection with C. jejuni was observed; up to 3% of cases may be followed by Reiter’s syndrome, or by a reactive arthritis. However, this hypothesis was excluded because of the lack of compatible clinical features (reactive arthritis is usually oligoarticular, asymmetric, and most frequently involves the knees, ankles, or wrists), and lack of improvement after antibiotic therapy for C. jejuni infection.11,12
The last hypothesis the authors have taken into consideration was EIM associated with intestinal flare. A wide range of EIMs can occur in up to 50% of patients with IBD, and their onset may precede the diagnosis of IBD or arise later, as single or multiple manifestations. The most frequent are spondyloarthritis, pauciarticular arthritis (directly associated with intestinal flare), and ankylosing spondylitis, independent of intestinal disease activity. Nevertheless, the patient’s symptoms did not completely fit diagnostic criteria for any of the above-mentioned manifestations. In light of sternoclavicular and temporomandibular joint involvement, the presence of fever and headache, and the absence of axial involvement, SAPHO syndrome was hypothesised.
Although SAPHO syndrome was initially considered to be a seronegative spondyloarthropathy, recent evidence suggests that it may be better described as primary inflammatory osteitis, within the spectrum of autoinflammatory diseases.13-15 The onset of the disease varies from infancy to late adulthood, with an average age between 30–40 years. The main clinical features are osteitis and hyperostosis, which typically involve the anterior chest wall (in particular sternocostal joints, sternoclavicular joints, and the costoclavicular ligament), followed by the axial skeleton, the long bones of the extremities, the irregular bones (such as mandible), and the peripheral joints. Skin involvement usually appears 1–2 years after skeletal changes, but can also be simultaneous, or arise after more than 20 years, and may or may not be present in patients with these osteoarticular features, especially in association with IBD.
The association of SAPHO syndrome with IBD was first reported by Kahn et al.4 in 1992, and subsequently in several case reports and case series.16-19 The prevalence of IBD among patients with SAPHO has been estimated to be from 8–13% in two large studies, with higher prevalence in ileocolic CD compared to ulcerative colitis. Typically, SAPHO occurs in young patients with CD who have colonic involvement; however, prevalence of this association might be underestimated among patients with IBD because of clinical similarities with other EIMs, and drug-related side effects.
The literature on SAPHO syndrome consists mainly of case reports, case series, or observational cohorts.20 Treatment, as for seronegative spondyloarthropathies, consists primarily of non-steroidal anti-inflammatory drugs, which, however, are ineffective in some cases. The second step involves disease-modifying antirheumatic drugs, such as methotrexate, sulfasalazine, and cyclosporin A, but even with these, a significant proportion of patients are unable to achieve remission. In the biologic era, anti-TNFα are considered the first choice treatment in patients with IBD and EIMs; however, more and more evidence is emerging on the efficacy and safety of alternative therapeutic options, such as ustekinumab, vedolizumab, and secukinumab, especially in patients with arthralgia.21,22 This may be of particular importance in SAPHO syndrome, since the IL-23/Th17 axis appears to be implicated in its pathogenesis, and an increase in Th17 cells in peripheral blood has been demonstrated.23 In the authors’ patient, who had a previous drug history of adalimumab failure, infliximab intolerance, and evidence of moderate-to-severe flare, together with severe endoscopic activity while on vedolizumab as a maintenance treatment, the swap to ustekinumab was proposed with sustained clinical remission, both on intestinal and articular manifestations.
CONCLUSION
This case underlines the importance of joint management between gastroenterologists and rheumatologists for patients with IBD with articular manifestation. In particular, the authors want to highlight the exciting challenge of differential diagnosis in those patients in which the same presentation can hide several clinical entities, even those as rare as SAPHO syndrome. A correct differential diagnosis guarantees the best clinical outcome, and to follow a therapeutic approach that should always consider the coexistence of intestinal disease and extraintestinal manifestations. In this case, ustekinumab proved to be an effective therapy in patients with CD with SAPHO syndrome, allowing the control of both intestinal and articular manifestations.







