Abstract
In chronic inflammatory disease, TNF-α is produced in high concentrations, leading to excessive inflammation and eventually organ damage. The advent of anti-TNF therapy in clinical practice 20 years ago represented a significant change in the management of immune-mediated inflammatory diseases (IMID). Common concerns regarding the safety profile of anti-TNFs include increased infection rates, associations with cancer, and safety in pregnancy. Regulatory authority guidelines to reduce risk include vaccination and screening for latent infections prior to treatment initiation. In general, pharmacovigilance and tailored medicine are the best methods for optimising anti-TNF therapy while minimising side effects. This mini review aims to summarise the current understanding of the safety profile of this drug class.
INTRODUCTION
Up to 10% of the Western population are affected by IMIDs,1 including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), spondyloarthritis, and inflammatory skin conditions such as psoriasis and hidradenitis suppurativa.2
IMIDs span the fields of gastroenterology, rheumatology, and dermatology, with increasing understanding of the overlapping pathogenesis between them. As such, patients with one IMID have an increased risk for developing other IMIDs.1 For example, patients with IBD have over five times higher risk of developing ankylosing spondylitis (AS) and over two times higher risk of developing RA compared with the general population.3,4 Similarly, psoriatic arthritis (PsA) can develop in up to 24.0% of patients with psoriasis5 and 3.3% of patients with hidradenitis suppurativa have IBD, a prevalence several fold higher than the general population.6 In Denmark, a nationwide survey indicated that 22.5% of patients with IBD had at least one concurrent IMID, most commonly psoriasis, asthma, Type 1 diabetes, or uveitis.7
ANTI-TNFS AS A THERAPY FOR IMMUNE-MEDIATED INFLAMMATORY DISEASES
In chronic inflammatory diseases, the cytokine TNF-α is produced in high concentrations, leading to excessive inflammation and eventually organ damage.8 Anti-TNFs, including monoclonal antibodies and competitive ‘decoy’ receptors, bind to TNF-α to inhibit its interaction with the TNF receptor9,10 and have been approved for the treatment of a wide range of IMIDs.10-19
The advent of anti-TNF therapy has led to significant progress for patients with chronic inflammatory disease20 and, to date, there are five anti-TNF agents approved in Europe and the USA for use in IMIDs: adalimumab, infliximab, golimumab, etanercept, and certolizumab pegol.10-19
The management of IMIDs often requires life-long treatment, and anti-TNF therapy has been used in clinical practice for over 20 years,21 making assessments of long-term safety possible.
SAFETY OF ANTI-TNF MEDICATION
Though the safety profile of anti-TNFs is generally considered to be acceptable in relation to the associated benefits,22,23 there remains concern22 regarding potential associations with infection, malignancy, and immunogenicity.21
Safety data should be assessed alongside the known risks of IMIDs themselves of concomitant medication such as non-steroidal anti-inflammatory drugs24 and of non-treatment of the condition in question.25 For example, inflammation is a known risk factor for cancer,24 and the risk of malignancy is higher in patients with IMID than in the general population.26 In patients with Crohn’s disease (CD), both disease activity and narcotic analgesics are associated with serious infections.27
Several studies have assessed overall mortality associated with anti-TNF treatment. Carmona et al.25 investigated the impact of anti-TNFs on the survival of patients with RA, and found that mortality from non-infectious causes is decreased in patients treated with anti-TNFs compared with patients with RA not exposed to anti-TNFs. The main reason for this decline was the reduction in mortality from cardiac causes.25 Similarly, Lima et al.,22 reported that the mortality rate in patients with CD treated with infliximab was similar to that in patients receiving other treatments. Regarding adalimumab, studies have reported no increased mortality rate associated with treatment of RA, AS, psoriasis, PsA, or CD compared with a matched control population.26 For patients with CD, mortality rates were similar between those treated and not treated with infliximab.28 In a 5-year, European CD registry, prednisone was associated with higher mortality (hazard ratio [HR]: 3.58 versus 1.22, respectively), whereas infliximab was not.29 In fact, in exposure-adjusted Poisson regression analyses, infliximab was actually associated with a lower risk of mortality compared with conventional therapy (relative risk [RR]: 0.39).29
Specific areas of concern regarding the safety of anti-TNFs in IMID are summarised in Figure 1, and reviewed individually below.
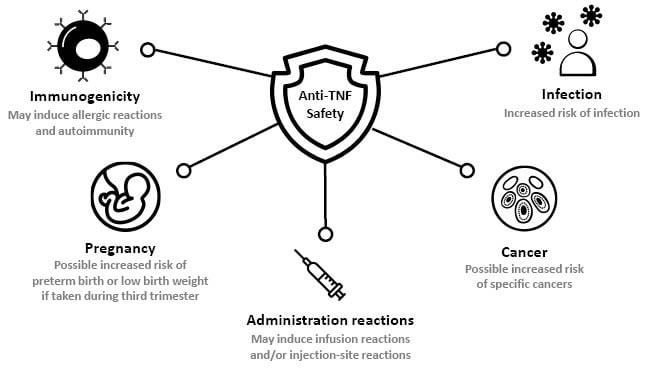
Figure 1: Summary of anti-TNF safety concerns in immune-mediated disease.
Increased Risk of Infection
One of the most studied aspects of anti-TNF safety is the potential increased risk of infection.
Comparisons of the infection rate between patients treated and not treated with anti-TNFs have generally reported an increased rate of infection with anti-TNF therapy. For example, a case control study using data from a 3-year French registry (n=45 cases) found that anti-TNF treatment for any indication was a risk factor for opportunistic infections, with an annual incidence of 0.15 per 100 person-years (PY).30 Similarly, a meta-analysis of real-word studies in patients with IBD indicated a median rate of serious infections of 3.9 events per 100 PY in patients receiving anti-TNF monotherapy versus a rate of 2.2 per 100 PY with immunomodulator monotherapy, corresponding to a 64% higher risk with anti-TNF therapy.31
In the USA, a retrospective cohort study using data from four large USA-based data systems found that new users of anti-TNFs for RA, IBD, psoriasis, PsA, or AS had an increased risk of non-viral opportunistic infections compared with patients initiating non-TNF-based therapy (adjusted HR: 1.6).32 Similarly, real-world registry data from North America (n=6,273) found that serious infections occurred at 2.2 events per 100 PY in patients treated with infliximab versus 0.9 events per 100 PY in patients with CD treated with other therapies.28
In Europe, an analysis of the real-world ENCORE registry (n=2,662) found that infliximab was associated with serious infections in patients with CD (HR: 1.64), with a rate of 2.7 events per 100 PY in patients treated with infliximab versus 1.7 events per 100 PY in patients who were not treated with infliximab.29 A real-world cohort study in Spain also reported that the rate of serious infections was higher in patients with RA who received anti-TNF treatment (n=4,459) compared with those who did not (n=789), with an RR of 1.6.25
The most common opportunistic infection that has been described with anti-TNF treatment is the reactivation of tuberculosis (TB) from its latent form.26 This is thought to relate to the role of TNF in the formation of granulomas, which restrict the growth of TB.8
The 2018 European Society of Clinical Microbiology and Infectious Diseases (ESCMID) consensus document suggests that anti-TNF therapy is associated with a two- to four-fold increase in the risk of TB reactivation.33 Before the advent of systematic screening and prophylactic treatment, reactivation rate during anti-TNF treatment was 1.5 events per 100 PY.26 This increased risk of TB has led all anti-TNF labels to include a warning that patients should be tested for latent TB prior to initiating treatment and, if this test is positive, treatment for TB should be initiated before starting anti-TNF therapy.10-19
Following the implementation of screening for TB prior to the initiation of anti-TNF treatment, the TB reactivation rate dropped sharply to a rate of 0.2 events per 100 PY.26 Consequently, real-world studies over the past few years have observed low rates of TB in patients treated with anti-TNFs. For example, patients with CD treated with infliximab had an active TB rate of 0.01 event per 100 PY;28 patients with RA treated with anti-TNFs had a rate of approximately 0.1 per 100 PY;34 and patients with RA, PsA, or AS treated with golimumab did not experience any reactivation of TB.35
Moreover, the risk of TB may differ between anti-TNF drugs. In an analysis of the British Society for Rheumatology Biologics Register (BSRBR) of patients with RA (n=10,712), treatment with monoclonal antibodies infliximab or adalimumab was associated with an increased risk of TB compared with treatment with the soluble TNF receptor, etanercept, with infection rates of 0.14, 0.14, and 0.04 events per 100 PY, respectively.34
Administration Reactions
Administration of all intravenous anti-TNF agents is associated with infusion reactions, which mainly occur in the first month of treatment, though the exact aetiology and pathogenesis of these responses are unclear.24,36 Infusion reactions to anti-TNFs can include pruritis, flushing, dyspnoea, chest discomfort, myalgia, nausea, and other symptoms.36 For example, in a retrospective analysis of patients with RA treated with infliximab over 9 years, 5.8% experienced an acute infusion reaction.37
Anti-TNF therapy is also associated with injection-site reactions, such as pain, swelling, or itching.10 In a meta-analysis of 6 studies of patients with AS, the risk of injection site reactions was significantly higher with anti-TNF therapy than with placebo (RR: 2.93).38 In paediatric patients (n=577), adalimumab treatment was associated with injection-site reactions in 37% of patients with juvenile idiopathic arthritis, 10% of those with psoriasis, and 22% of those with CD.39 Most events were mild or moderate in severity.
Immunogenicity
Although TNF-α drives an inflammatory response to infection or injury, it also limits the extent and duration of this response once the trigger has been neutralised, and this characteristic may partly explain why anti-TNF therapy can sometimes induce or exacerbate immune reactions.21
Both local and systemic allergic reactions have been described with the use of anti-TNF therapy in IMIDs and, in some instances, desensitisation approaches or switching to an alternative anti-TNF agent has been successful.26 For example, approximately 7.4% of patients may experience mild hypersensitivity reactions.40
The onset of autoimmune syndromes has also been described in patients treated with anti-TNFs. The incidence of drug-induced systemic lupus erythematosus, related to the development of auto-antibodies was found to be 1.3% in a systematic review of patients with any condition treated with anti-TNFs.41 Cases of psoriasis have also been reported in patients receiving anti-TNF therapy; a review concluded that cases mainly appear to be de novo psoriasis rather than aggravation of pre-existing disease, and eruptions are most often pustular lesions on the palms of the hand and/or soles of the feet.42 In a retrospective IBD study, 29% of patients (264 out of 917) treated with anti-TNFs developed skin lesions, most commonly eczema, but most of these patients did need to discontinue therapy.43
There have been rare reports of patients developing demyelinating diseases during treatment with anti-TNFs, though this phenomenon is not well understood.24,44 Regulatory authorities recommend that patients are assessed for the individual risk of developing demyelinating disorders prior to treatment initiation.10-19
Cancer
All anti-TNFs currently include a black box warning for malignancy.10-19 However, the relationship between anti-TNFs and cancer is controversial, since TNF-α exerts both pro- and anti-cancer effects.45 Many patients treated with anti-TNFs concomitantly take other immune-suppressing medications, some of which have also been associated with cancer,22,46 and inflammation is itself a known risk factor for malignancy, making it difficult to establish a causal relationship.24
There are multiple examples of evidence that do not support an association between anti-TNFs and cancer. For example, a meta-analysis of 16 studies in patients with IMIDs (n=11,702) found that malignancy rates were similar between patients receiving anti-TNF therapy (3.4 per 100 PY), immune-modulator therapy (3.6 per 100 PY), and no immunosuppression (3.8 per 100 PY).46 Similarly, a meta-analysis of 33 randomised controlled trials involving patients with RA found that treatment with anti-TNF treatment over 2 years was not associated with an increased risk of cancer compared with placebo (odds ratio [OR]: 0.93).45 Analysis of 5-year follow-up data from a European CD registry found that infliximab was not associated with malignancy when compared with conventional therapy (HR: 1.44).29 In addition, 13-year follow-up data from a large North American CD registry (n=6,273) indicated that malignancy rates were similar between patients treated and not-treated with anti-TNFs (0.69 and 0.71 events per 100 PY, respectively).28
There is also evidence suggesting that anti-TNFs do not increase the risk of malignancy in patients with a history of cancer. A meta-analysis of 9 observational studies found that the pooled RR of new or recurrent cancer in this population was not significantly different between patients treated and not treated with anti-TNFs (incidence rate ratio: 0.90).47
Interestingly, in one real-world study, anti-TNF treatment was actually reported to reduce the rate of malignancy. A real-world cohort study in Spain reported that the rate of malignancy was higher in patients with RA who did not receive anti-TNF treatment (n=789) compared with those who did (n=4459), with an RR of 2.9.25
However, a trend towards an increased risk of non-melanoma skin cancer has been observed in patients with RA who have been treated with anti-TNFs versus placebo (OR: 1.37).45 In a large retrospective IBD study, the use of anti-TNFs appeared to increase the risk of melanoma.48 A significantly increased risk of lymphoma was observed in patients with IBD treated versus not treated with anti-TNFs in France, though the incidence rate was low (0.04 versus 0.03 events per 100 PY, respectively).49 A similar study in Denmark, however, did not find any evidence of this association.50
Finally, in a recent non-inferiority trial in patients with RA, the incidence of cancer was found to be lower with anti-TNF treatment (N=42) than with JAK inhibitor (N=122) treatment over a median follow-up of 4 years (2.9% versus 4.2%).51
Pregnancy
Anti-TNF therapy is increasingly continued during pregnancy to prevent flares in patients with IMIDs, which have been associated with an increased risk of miscarriage, preterm birth, and low birth weight.52 A trend towards drug-specific harm has been reported, in terms of congenital malformations and preterm birth. However, IMIDs themselves may also increase the risk of adverse pregnancy outcomes.52,53
Data on the safety of anti-TNFs during pregnancy are conflicting.52,53 In a meta-analysis of 13 studies involving females with IMIDs, the risk of preterm birth, spontaneous abortion, and low birth weight was comparable between patients treated and not treated with anti-TNF.54 In RA, anti-TNFs was not associated with an increased risk of congenital malformation compared with RA controls, but more than half of the females in this study discontinued therapy at some point during their pregnancy.26
In contrast, a recent questionnaire-based study of 153 females with active IBD indicated that continuation of anti-TNF therapy after 30 weeks of pregnancy was associated with reduced birth weight.55 In support of this finding, a recent meta-analysis of females with IBD found that exposure to anti-TNFs during pregnancy was associated with an increased risk of preterm birth (OR: 1.66), and low birth weight (OR: 1.49) compared with similar controls.However, this increased risk was not observed in a group of females with RA or psoriasis.52
Guidelines indicate that females with active psoriasis should be treated appropriately during pregnancy, and that anti-TNF treatment should be discontinued before the third trimester.53 Similarly, it is recommended and appears to be reasonably safe to use anti-TNFs in rheumatic diseases and IBD until the 24th week of pregnancy.56
MANAGING SAFETY CONCERNS
The increased risk of new infections or reactivation of latent infections associated with anti-TNF treatment should be addressed by screening for TB and other severe opportunistic infections such as hepatitis B prior to the initiation of therapy.10-19
Several studies have indicated that concomitant steroid treatment poses an additional risk factor for infections in patients receiving anti-TNFs,29,30,32,57 suggesting that the use of this combination should be carefully considered and monitored by the treating clinician.
Some studies have indicated that etanercept is associated with a reduced risk of infection compared with infliximab or adalimumab,30 and it may be less likely to reactivate TB,38 indicating that this anti-TNF agent could be particularly beneficial to patients at higher risk of infections.
In pregnancy, certolizumab pegol may have potential beneficial effects compared with other anti-TNF agents, because this antibody lacks an IgG Fc region and, therefore, shows only minimal transfer across the placenta.51,58 For this reason, certolizumab pegol is considered safe to continue throughout pregnancy.52
Interestingly, infliximab treatment in patients with IBD has been associated with a reduced risk of developing a secondary IMID, implying a potential additional protective effect of anti-TNF treatment.7
CONCLUSIONS AND SUMMARY
Anti-TNF therapy in patients with IMIDs is not associated with increased mortality compared with alternative treatments.22,25,26,28,29 Indeed, there is some evidence that it may be associated with decreased mortality.29
However, anti-TNFs are associated with increased risk of infection,25,28-32 which may be addressed with adequate screening prior to treatment initiation and monitoring during therapy.10-19 The association with malignancy is more controversial; although anti-TNFs currently include black box warnings for malignancy,10-19 recent studies have not generally supported an increased overall risk of malignancy with anti-TNF treatment.28,29,45,46 Nevertheless, there are some reports that suggest an increased risk of specific cancers, such as non-melanoma skin cancer in patients with RA and lymphoma or melanoma in patients with IBD.45,48,49 In pregnancy, anti-TNFs are generally considered safe to use during the first and second trimesters. However, with the exception of certolizumab pegol, it is recommended that treatment is discontinued before the third trimester, when these agents will be actively transported across the placenta.52,53,58
For both clinicians and their patients, pharmacovigilance and tailored medicine are the best methods for optimising anti-TNF therapy while minimising side effects.24

![EMJ Gastroenterology 11 [Supplement 3] 2022 Feature Image](https://www.emjreviews.com/wp-content/uploads/2022/07/EMJ-Gastroenterology-11-Supplement-3-2022-Feature-Image-940x563.png)





