Abstract
Endoscopic retrograde cholangiopancreatography (ERCP) has evolved from a diagnostic modality to a therapeutic tool for various biliary and pancreatic diseases. The major reason for this evolution is the risk of post-ERCP pancreatitis (PEP) and the availability of safer non-invasive imaging modalities. PEP is the most common and dreaded complication after ERCP, with significant morbidity and mortality. Several pharmacological therapies and modifications to endoscopic techniques have been evaluated in different clinical settings to prevent PEP; however, except for few, evidence to support the practice of most is poor. Rectal non-steroidal anti-inflammatory drugs, aggressive hydration with lactated Ringer’s solution, and prophylactic pancreatic duct stenting are some of the preventive measures strongly recommended by endoscopic societies, although the quality of evidence is low to moderate. Evidence in support of a combination of rectal non-steroidal anti-inflammatory drugs and aggressive hydration is emerging. Despite the recent developments in the prevention strategies, the risk of PEP remains substantial. Therefore, proper risk stratification of patients and the development of better risk mitigation strategies are the need of the hour.
INTRODUCTION
The therapeutic technique of endoscopic retrograde cholangiopancreatography (ERCP) has evolved as a therapeutic endoscopic technique for various benign and malignant conditions of the pancreato-biliary system. Post-ERCP pancreatitis (PEP) is the most common and dreaded complication after ERCP, with significant morbidity and mortality. For the same reason, the use of ERCP for diagnostic indications has virtually diminished with the emergence of safer, non-invasive imaging modalities such as magnetic resonance cholangiopancreatography and endoscopic ultrasound. The incidence of PEP ranges widely between 3.5% and 9.5%, and mortality between 0.1% and 0.7%.1,2 A recent nationwide study suggested an increasing rate of hospital admission and mortality in the USA (mortality: 2.8% in 2011 and 4.4% in 2017).3 Since Freeman et al. studied the risk factors of PEP prospectively for the first time in 1996, there have been a plethora of publications over the past two decades in this field.4 Risk stratification of patients, based on the number and nature of risk factors, is crucial for proper patient selection before ERCP and for the initiation of appropriate preventive measures in a timely fashion. Although PEP is mild in the majority of the cases, mortalities have been reported and, therefore, prevention is the best strategy to save these patients.5
In this review, the authors discuss the definition, severity assessment, risk factors, and prevention of PEP.
DEFINITION AND SEVERITY ASSESSMENT
PEP is defined by the 1991 consensus criteria as new-onset or worsened abdominal pain, with more than three-fold elevation in serum amylase or lipase at more than 24 hours after ERCP, requiring hospital admission or prolongation of a planned admission.6 Although, this definition is widely accepted, minor variations in the minimum duration of hospital stay have been proposed.7 Cross-sectional imaging of the pancreas is generally not required to make a diagnosis of PEP; however, imaging is required to grade the severity of pancreatitis, according to the revised Atlanta classification.8
The consensus criteria and the lexicon of adverse events proposed by the American Society of Gastrointestinal Endoscopy (ASGE) grade the severity of PEP based on the length of hospital stay.6,7 In addition, the ASGE lexicon also considers the requirement of intensive care unit admission, radiological or surgical intervention, permanent disability, and death. The revised Atlanta classification appears to be more specific for PEP and stratifies the patients according to the presence of local complications and the duration of organ failure.8 A recent multicentre study of 387 patients with PEP found that the revised Atlanta criteria, compared with the consensus criteria, had a superior sensitivity, specificity, and positive predictive value in predicting mortality.9 The length of hospital stay is dependent on multiple factors and may not reflect the severity of the disease, and is often influenced by concomitant diseases or comorbidities.
RISK FACTORS FOR POST-ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY PANCREATITIS
The stratification of patients undergoing ERCP is paramount for implementing appropriate preventive strategies against PEP. Risk factors for PEP may be classified as definite or likely based on the level of evidence in the published literature (Table 1).5 A patient is considered to be at high-risk for PEP if one definite or two likely patient- or procedure-related risk factors are present. Among the patient-related definite risk factors, including female gender, sphincter of Oddi dysfunction (SOD), previous pancreatitis, and previous PEP, have been shown to be consistently associated with PEP.12-14 In addition, younger age (<60 years), normal serum bilirubin, and non-dilated common bile duct have been confirmed to be independent risk factors for PEP in prospective multicentre studies.4,11,15-17 Difficult cannulation of Vater’s papilla and major pancreatic duct (PD) injection are the major procedure-related definite risk factors for PEP.13,14 Other procedure related risk factors include multiple attempts at cannulation and pancreatic guidewire passage.18
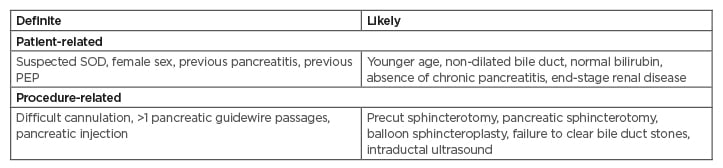
Table 1: Risk factors for post-endoscopic retrograde cholangiopancreatography pancreatitis.5,10,11
PEP: post-endoscopic retrograde cholangiopancreatography pancreatitis; SOD: sphincter of Oddi dysfunction.
Contrast to the popular belief, needle knife precut sphincterotomy is not associated with increased risk of PEP. On the contrary, early precut appears to be protective against PEP in difficult biliary access. Two recent meta-analyses have shown that early precut, compared with persistent cannulation attempts, can significantly decrease the incidence of PEP (relative risk [RR]: 0.29–0.57) in difficult biliary access.19,20 In selected high-risk cases, primary needle knife fistulotomy appears to reduce the risk of PEP when compared with conventional cannulation methods.21
Alternatively, in difficult biliary access, the double guidewire (DGW) technique has been proposed to increase biliary cannulation rates. This technique involves placement of a guidewire deep into the PD, followed by attempts to cannulate the common bile duct using a second guidewire. However, the DGW technique increased the risk of PEP in a recent meta-analysis (RR: 1.98; 95% confidence interval [CI]: 1.14–3.42) and the risk was reduced by concomitant PD stenting.22
Although biliary balloon sphincter dilation is a risk factor for PEP,14 large-balloon dilation and dilation for a longer duration (>3 minutes) could reduce the incidence of PEP as insufficient dilation increases the use of mechanical lithotripsy and puts stress on the papilla during stone removal.23,24 On the contrary, a recent multicentre, randomised trial concluded that the incidence of PEP increased significantly after dilatating for 300 seconds when compared with dilatating for shorter durations, i.e., 30, 60, and 180 seconds (15% versus 7%, 8%, and 9%).25 The authors concluded that 30 seconds dilatation time was optimal with regard to the incidence of PEP.
With regard to hospital volume and the experience of the endoscopist, a recent study showed that PEP was more common when the procedure was performed by less experienced endoscopists (<200 procedures) (odds ratio [OR]: 1.630; 95% CI: 1.050–2.531).26 However, a recent meta-analysis failed to demonstrate a significant difference in the risk of PEP between high- and low-volume endoscopists (<40 /year and >40 /year procedures) or centres (<200 /year and >200 /year procedures), although only three studies included in the analysis reported PEP specifically.27
PATHOPHYSIOLOGY
The aetiopathogenesis of PEP is multi-factorial and includes an increase in pancreatic ductal pressure and spasm of the SO, causing mechanical obstruction. A SO spasm may directly result from mechanical trauma or indirectly due to hypersensitive sphincter as in SOD. Irrespective of the underlying cause, these inciting factors ultimately initiate an inappropriate activation of proteolytic enzymes and cytokine release, leading to a vicious inflammatory cycle.
PREVENTION OF POST-ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY PANCREATITIS
Prevention of PEP includes pharmacological, endoscopic, and combined approaches. Since rectal non-steroidal anti-inflammatory drugs (NSAIDs) have opened a new era in this field, the authors discuss the current available preventive strategies pertinent to advances in the last decade.
Wire-Guided Cannulation
There are two main techniques of cannulation: contrast-assisted and wire-guided. Inadvertent contrast injection into the PD is a well-known risk factor for PEP. On the other hand, guidewire-assisted cannulation has been shown to increase the success rates of cannulation and reduce the risk of PEP when compared with the contrast-assisted cannulation technique.28 In addition, inadvertent guidewire cannulation into the PD may facilitate biliary cannulation by the DGW technique. A prophylactic PD stent should be placed to mitigate the risk of PEP associated with this technique.29 In expert hands, early use of alternate cannulation techniques like PD stenting and precut sphincterotomy are equally effective in achieving cannulation, as well as reducing the risk of PEP. In practice, a hybrid technique (guidewire plus contrast) is often utilised where a small volume of contrast guides the path of the guidewire. Although the hybrid technique may facilitate biliary cannulation, the risk of PEP appears to be unchanged when compared with exclusive wire guided cannulation.30
The ASGE guidelines recommend that physicians who perform ERCP be facile with procedural techniques that reduce the risk of pancreatitis (i.e., wire-guided cannulation, prophylactic pancreatic duct stenting).31
Pharmacological Prevention
Non-steroidal anti-inflammatory drugs
NSAIDs exert their anti-inflammatory action by inhibition of cyclo-oxygenase (COX), especially an inducible form of COX-2. The hypothetical mechanisms of COX-2 inhibition in ameliorating pancreatitis include reduction in prostaglandin synthesis and pancreatic oedema, and suppression of proinflammatory nuclear transcription factor κB.32 The effectiveness of NSAIDs in preventing PEP is affected by the route and timing of administration.
Among the non-selective COX inhibitors, indomethacin and diclofenac have been extensively studied in recent trials (Table 2). Oral and intramuscular routes of administration have been shown to be ineffective in the prevention of PEP for unclear reasons.44 In a randomised study including 207 patients, there was no difference in the incidence of PEP between oral diclofenac and placebo groups (16.2% versus 16.7%).45 In another multicentre, randomised study including 216 patients, the combination of udenafil and aceclofenac failed to reduce the incidence of PEP over placebo.46 Similar to oral route, prophylactic intramuscular diclofenac has been found to have no preventive effect on PEP.47 Although, the bioavailability (80–100%) is excellent with both the routes (oral and rectal) and their plasma concentrations peaking at 60–90 minutes, peak plasma concentration is more sustained (>2 hours) and declines slowly after rectal administration compared with oral and intramuscular administration.48 Sustained plasma concentration after rectal NSAIDs may play a key role in preventing PEP.49
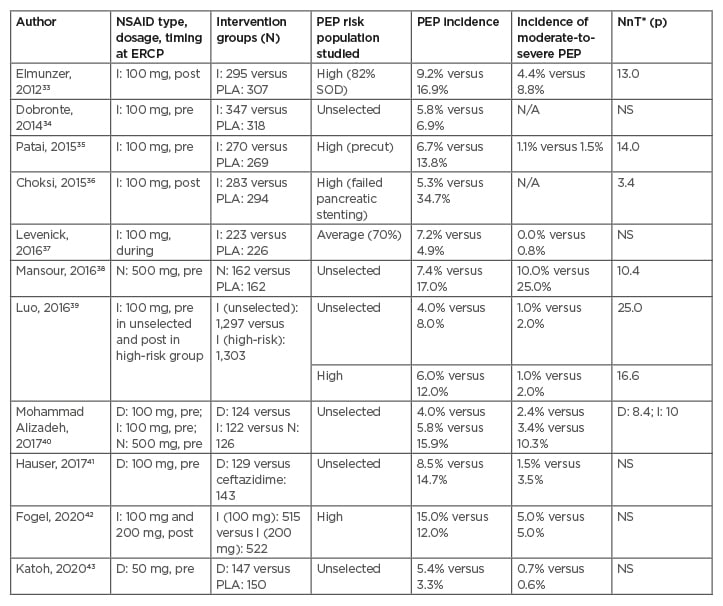
Table 2: Randomised controlled trials of rectal non-steroidal anti-inflammatory drugs, conducted between 2010 and 2020 for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis.
Only trials with >200 patients were included.
*Value reported only when p<0.05.
D: diclofenac; ERCP: endoscopic retrograde cholangiopancreatography; I: indomethacin; N: naproxen; NSAID: non-steroidal anti-inflammatory drug; PEP: post-ERCP pancreatitis; PLA, placebo; post: after ERCP; pre: before ERCP; N/A: not available; NnT: number needed to be treated; NS: non-significant: SOD: sphincter of Oddi dysfunction.
In contrast to diclofenac, oral indomethacin is not subject to significant first pass metabolism. Therefore, indomethacin may be effective by oral route, theoretically. However, this hypothesis needs to be substantiated by quality studies. Two meta-analyses that evaluated the optimal timing of administration of rectal NSAIDs (before or after ERCP) have given conflicting results.50,51
In a large multicentre trial, rectal indomethacin administration before ERCP, compared with after ERCP, was more effective in reducing the incidence of PEP (6% versus 12% [RR: 0.47; 95% CI: 0.27–0.82]) in high-risk patients.39 Since the plasma concentration peaks at 90 minutes after rectal administration, the optimal timing of rectal NSAIDs administration may be 90 minutes before ERCP. Nevertheless, the timing of rectal NSAIDs remains an active area for research. In a recent meta-analysis (21 randomised clinical trials [RCT], 6,854 patients), rectal NSAIDs were more effective than placebo in reducing the overall incidence of PEP (risk difference: -0.07; 95% CI: -0.10–-0.04; number needed to be treated [NnT]: 20; p<0.001).52 Although rectal NSAIDs effectively prevented mild PEP, the effect on moderate-to-severe PEP has not been consistent.35,39 A recent meta-analysis (19 RCTs, 5,031 patients) confirmed that rectal NSAIDs were associated with significant reduction in the risk of moderate-to-severe PEP.53 In regard to PEP risk stratification, prophylactic effect of rectal NSAIDs was consistent in the group of patients who are at high-risk;33,35,36 however, results were not reproducible in those at average (not fulfilling the high-risk criteria) and low-risk.34,37,41
Universal prophylaxis using rectal NSAIDs for PEP across all risk groups has been a matter of debate. In a recent multicentre, randomised trial, preprocedural administration of rectal indomethacin in unselected patients reduced the overall occurrence of PEP.39 In addition, considering the relatively low cost, safety profile, and ease of administration of rectal NSAIDs, it is reasonable to administer rectal NSAIDs in all patients undergoing ERCP. The optimal dose of rectal indomethacin or diclofenac is 100 mg. While increasing the dose does not appear to confer additional benefit,42 lower doses are ineffective in preventing PEP.43 Administration of NSAIDs is contraindicated in pregnant women >30 weeks of gestation, in patients with history of Stevens–Johnson syndrome, and in those with impaired renal function, particularly taking antihypertensive drugs.5
The European Society of Gastrointestinal Endoscopy (ESGE) guideline recommends routine rectal administration of 100 mg of diclofenac or indomethacin immediately before ERCP in all patients without contraindications to NSAID administration (strong recommendation, moderate quality evidence).5 The ASGE guidelines recommend rectal NSAIDs in high-risk individuals (moderate evidence).31 In average-risk individuals, rectal indomethacin may reduce the risk and severity of PEP (low quality evidence).
Sublingual nitrates
Nitrate is a smooth muscle relaxant and is believed to prevent PEP by inhibiting SO spasm and increasing pancreatic parenchymal blood flow. The prophylactic role of nitrates was confirmed in a meta-analysis (12 RCTs, 2,649 patients). Although, glyceryl trinitrate (GTN) significantly reduced the overall incidence of PEP (RR: 0.70; 95% CI: 0.52–0.87) the incidence of moderate-to-severe PEP was not affected. Subgroup analysis revealed that sublingual route of GTN administration was more effective than transdermal and topical routes in preventing PEP, particularly in those who are at high-risk.54 More recently, the effect of combination of rectal NSAIDs (indomethacin or diclofenac) and sublingual isosorbide dinitrate (5 mg) was evaluated in two RCTs that largely involved patients who were at high-risk (70–80%). The combination therapy administered before ERCP was superior to rectal NSAIDs alone in preventing PEP (NnT: 12–26).55,56 Transient hypotension was observed in up to 8% of patients in the combination group.56 Sublingual nitrates for the prevention of PEP should be considered before ERCP in patients who are at high-risk, in whom rectal NSAIDs and aggressive hydration are contraindicated.
The ESGE suggests the administration of 5 mg sublingual GNT before ERCP in patients with a contraindication to NSAIDs or to aggressive hydration for the prevention of PEP (weak recommendation, moderate evidence).5
Somatostatin and protease inhibitors
Somatostatin and protease inhibitors theoretically prevent PEP by inhibiting the activation of pancreatic proteolytic enzymes. Somatostatin, when administered as a long-term infusion (0.25 mg/hour, intravenous injection for ≥10 hours), initiated 30 minutes–1 hour before ERCP, was found to be superior to short-term (≤4 hours), or bolus injection in reducing the overall incidence of PEP. However, in a recent meta-analysis (15 RCT, 4,943 patients), the risk reduction was marginal (OR: 0.68; 95% CI: 0.47–0.98) compared with placebo, and the effect was largely limited to patients who are at high-risk (OR: 0.54; 95% CI: 0.34–0.86).57
Nafamostat, a potent protease inhibitor, is widely used in the Eastern countries for the prevention of PEP. Although nafamostat reduced the overall risk of PEP in two meta-analyses, requirement of intravenous infusion (for at least 6 hours), high cost, and the lack of benefit in patients who are at high-risk preclude its routine application in clinical practice.58,59 In a recent multicentre, randomised trial, nafamostat was not effective in preventing PEP, regardless of the timing of administration.60 Octreotide (somatostatin analogue) and less potent protease inhibitors such as gabexate and ulinastatin were found to be ineffective in preventing PEP.58,61
The ESGE has no recommendation about the use of somatostatin and does not recommend protease inhibitors for PEP prophylaxis (strong recommendation, moderate evidence).5
Aggressive Intravenous Hydration
Intravenous fluid resuscitation using lactated Ringer’s (LR) solution is the mainstay of treatment in the initial phases of acute pancreatitis, irrespective of the aetiology. Aggressive peri-ERCP hydration prevents haemoconcentration and restores pancreatic microcirculation, thereby minimising the risk of pancreatitis and its subsequent complications. Aggressive hydration with LR solution, compared with standard hydration, has been associated with lower incidence of PEP and moderate-to-severe PEP (NNT: 6–18) in groups of patients who are average- to high-risk62-64 (Table 3). A recent RCT (395 patients) reported that aggressive hydration with LR solution, but not with normal saline, significantly reduced the incidence of PEP compared with standard hydration.65 Henceforth, the protective effect of hydration against PEP may be specific to type and volume of the fluid. The total periprocedural fluid volume administered in aggressive hydration regimens is 35–45 mL/kg over 8 hours in contrast to 12–15 ml/kg in standard regimens. Adverse events due to fluid overload is observed in 1–2% of patients receiving aggressive hydration and the risk increases in older patients due to undiagnosed cardiac or renal comorbidities.65 The effect of combining aggressive hydration with rectal NSAIDs is not clear. Two out of three RCTs that evaluated the combination of aggressive hydration and rectal NSAIDs found that the combination was superior to rectal NSAIDs or hydration alone in reducing the overall incidence of PEP.66,68,69 Aggressive hydration with a LR solution should be considered in patients who are at high-risk in combination with rectal NSAIDs, or in those with contraindications to NSAIDs for prevention of PEP.
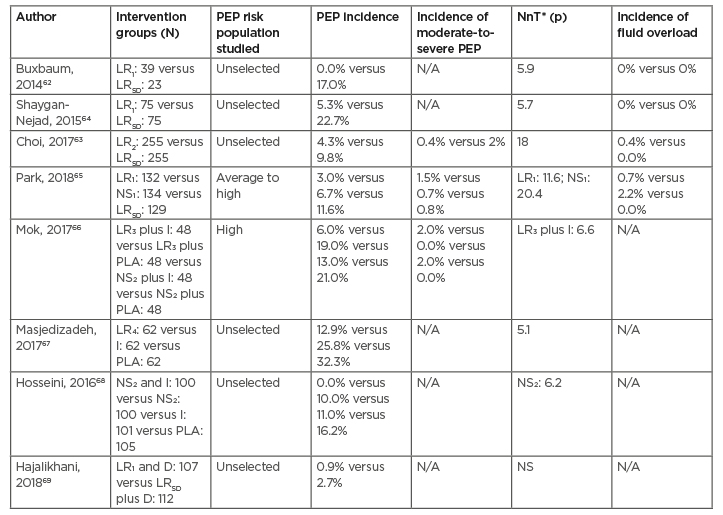
Table 3: Randomised controlled trials of aggressive hydration, with or without rectal non-steroidal anti-inflammatory drugs for the prevention of post- endoscopic retrograde cholangiopancreatography pancreatitis.
*Value reported only when the p=<0.05.
LR1: 3 ml/kg/hour during ERCP, 20 ml/kg bolus, and 3 ml/kg/hour for 8 hours after.
LR2: 10 ml/kg bolus before ERCP, 3 ml/kg/hour during and for 8 hours after, and 10 ml/kg bolus after ERCP.
LR3: 1 litre bolus over 30 minutes before ERCP.
LR4: 20 ml/kg bolus and 3 ml/kg/hour for 8 hours after ERCP.
LRSD: 1.5 ml/kg/hour during ERCP and for 8 hours after.
NS1: 3 ml/kg/hour during ERCP, 20 ml/kg bolus, and 3 ml/kg/hour for 8 hours after.
NS2: 1 litre before ERCP, 2 litres during, 16 litres after ERCP.
D: rectal diclofenac; ERCP: endoscopic retrograde cholangiopancreatography; I: rectal indomethacin; LRSD: lactated Ringer’s solution, standard hydration regimen; LR1-4: lactated Ringer’s solution, aggressive hydrogen regimens; N/A: not available; NnT: number needed to be treated; NS: non-significant; NS1,2: normal saline solution, aggressive hydration regimens; PEP: post-ERCP pancreatitis; PLA: placebo.
The ESGE recommends aggressive hydration with a LR solution (3 mL/kg/hour during ERCP, 20 mL/kg bolus after ERCP, and 3 mL/kg/hour for 8 hours after ERCP) in patients with contraindication to NSAIDs provided that they are not at risk of fluid overload and a prophylactic PD stent is not placed (strong recommendation, moderate evidence).5 The ASGE guidelines suggest periprocedural intravenous hydration with lactated ringers, when feasible, to decrease the risk of PEP (very low quality of evidence).31
Prophylactic Pancreatic Duct Stenting
PD stenting using small calibre plastic stents reduces the risk of PEP by relieving the obstruction at the level of ampulla of Vater. Recent meta-analyses reported a significant reduction in the overall incidence of PEP in patient groups who are unselected (OR: 0.21–0.25) and at high-risk (OR: 0.27–0.41), undergoing prophylactic pancreatic stenting compared with no stenting (NNT: 5–14).70,71
In addition, prophylactic pancreatic stenting markedly decreased the occurrence of moderate-to-severe PEP.72,73 The majority of trials of prophylactic pancreatic stenting in the rectal NSAIDs era (after 2010) have been carried out in patients who are at high-risk (Table 4). Given the high efficacy rate of rectal NSAIDs in preventing PEP, prophylactic pancreatic stenting should be limited to patients who become high-risk for PEP during ERCP, particularly in instances such as repeated inadvertent guidewire insertion into the PD and during the DGW technique of biliary cannulation.74 On the contrary, the risk of PEP increases after failed attempts of pancreatic stenting.80 The combination of rectal NSAIDs and pancreatic stenting have not been shown to be superior to either approach alone.79,81
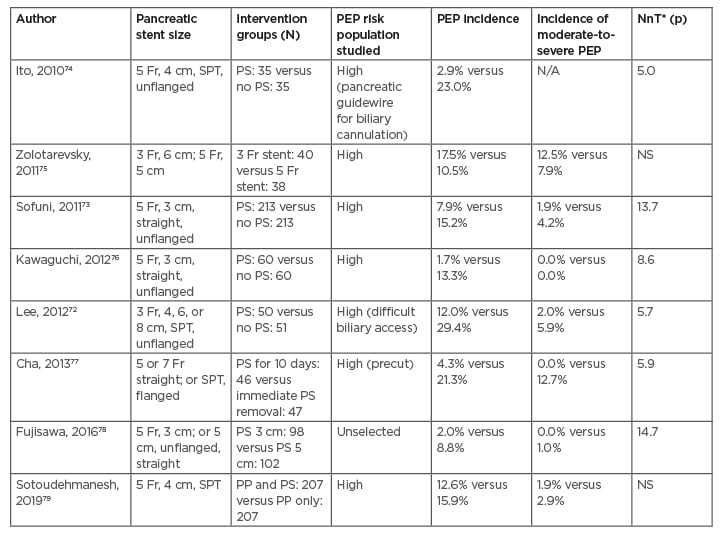
Table 4: Randomised controlled trials of prophylactic pancreatic duct stenting, with or without rectal non-steroidal anti-inflammatory drugs, conducted between 2010 and 2020 for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis.
*Value reported only when the p=<0.05.
Rectal indomethacin: 100 mg.
Sublingual isosorbide dinitrate: 5 mg.
Lactated Ringer’s solution: 6 ml/kg/hour during ERCP, 20 ml/kg bolus, and 3 ml/kg/hour for 8 hours after.
ERCP: endoscopic retrograde cholangiopancreatography; N/A: not available; NnT: number needed to be treated; NS: non-significant; PEP: post-ERCP pancreatitis; PP: pharmacological prophylaxis; PS: pancreatic stent; SPT: single pigtail stent.
In regard to the diameter and length of pancreatic stents, larger (5 Fr) and shorter (3 cm) stents are more efficacious than smaller (3 Fr) and longer (5 cm) stents in preventing PEP.75,78 Besides, stents with pigtail on the duodenal side and unflanged stents are preferred to prevent intraductal migration and to facilitate spontaneous elimination, respectively. A pancreatic stent should stay in place for at least 24 hours, since immediate removal of the stent after ERCP provides no protection against PEP.77 The majority of the small calibre pancreatic stents pass spontaneously within 4 weeks.
The position of the stent should be evaluated at 5–10 days of placement, using an abdominal X-ray, and should be removed endoscopically if retained. It is important to remove retained pancreatic stents in a timely fashion in order to reduce stent-induced ductal changes, including strictures.
The ESGE recommends prophylactic PD stenting in selected patients who are at high-risk for PEP (inadvertent guidewire insertion, opacification of the PD, or DGW calculation [strong recommendation, moderate evidence]).5 The ASGE recommend PD stenting to reduce the incidence and severity of PEP in individuals who are at high-risk.31
Topical Epinephrine Spray
Topical epinephrine spray over the papilla has been proposed to prevent PEP by reducing papillary oedema and PD outflow obstruction. Although initial reports were encouraging,82,83 two recent multicentre RCTs found that the combination of rectal indomethacin and topical spraying of epinephrine, compared with rectal indomethacin alone, did not reduce the incidence of PEP;84,85 indeed, one of the trials was prematurely terminated as the combination strategy increased the risk of PEP.85
The ESGE does not recommend topically administered epinephrine onto the papilla for PEP prophylaxis (strong recommendation, moderate evidence).5
Combined Prophylaxis
Several preventive strategies have been conclusively proven to be useful in the prevention of PEP. Nevertheless, the incidence of PEP refuses to reach an absolute zero. Since the mechanism of PEP prevention may be different among various methods, it seems prudent to combine different preventive strategies to optimise the outcomes. In this regard, the combination of rectal indomethacin and topical epinephrine have not been found to further reduce the risk of PEP when compared with rectal indomethacin alone.86 On the contrary, the combination was found to increase the risk of PEP over indomethacin alone in one randomised study.85 The proposed hypothesis for this paradox is reduction in the local concentration of indomethacin due to vasoconstriction induced by epinephrine, and possible activation of phospholipase A2, thereby antagonising the effect of indomethacin.
Another multicentre, randomised trial compared the effect of combined prophylaxis with aggressive hydration and rectal NSAIDs versus NSAIDs alone.87 There was no difference in the incidence of PEP between both the groups (8% combined versus 9% rectal NSAIDs). In tune with these studies, the combination of rectal NSAIDs and a PD stent was not superior to either approach alone in a systematic review and network meta-analysis.81
In contrast to the non-superior results of combined prophylaxis in the aforementioned studies, the combination of rectal diclofenac and sublingual isosorbide dinitrate was found to be superior to rectal diclofenac alone in preventing PEP in a recent multicentre study (5.6% combined versus 9.5% rectal diclofenac).54 Barring this study, there is limited data to support the role of combined prophylaxis in preventing PEP.
PUTTING IT ALL TOGETHER
The risk stratification of patients into low- and high-risk types enables the physician to plan and implement preventive strategies for PEP. However, the absence of risk factors does not guarantee the complete avoidance of PEP. Therefore, universal prophylaxis is recommended by most experts.
The cornerstone of PEP prophylaxis includes rectal NSAIDs, prophylactic PD stenting, and aggressive hydration using LR. These preventive modalities have stood the test of time and the evidence of their efficacy has been reproduced in multiple quality studies. The evidence of combination strategies (stent plus NSAIDs or LR) appear appealing due to different mechanisms of action. However, quality evidence is lacking regarding the superiority of the combination approach versus rectal NSAIDs alone. Nevertheless, planned or unplanned use of combination strategies is not uncommon in routine clinical practice.
In the authors’ unit, rectal NSAIDs are administered in unselected patients who undergo ERCP. Aggressive hydration is initiated in high-risk patients unless contraindicated. In selected patients who are at high-risk, especially those with repeated (>1) inadvertent insertion of a guidewire into the PD and those with contraindications to aggressive hydration and rectal NSAIDs, a prophylactic pancreatic stent is placed to prevent PEP as well as facilitate biliary cannulation. It is important to note that overzealous attempts at cannulation and deep access of the PD with a guidewire may be counterproductive and should be avoided. Once the guidewire is in the PD, the authors perform no more than two gentle attempts for deep PD access.
Post-ERCP, the authors continue with intravenous hydration with RL. Finally, preventive strategies for PEP are not fool-proof and, therefore, vigilance is required after ERCP as well. In cases with clinical suspicion of PEP, the authors prefer to restart aggressive hydration, pending the results of pancreatic enzyme assays.
SUMMARY
ERCP has been the cornerstone of treatment for multiple biliary and pancreatic diseases. The risk of PEP has pushed the utilisation of ERCP predominantly for therapeutic purposes. Despite the recent advances in this field, it may be difficult to predict the severity of pancreatitis. Consequently, it may be best to prevent PEP by using one or more preventive strategies.
Several preventive methods have been rigorously evaluated over the last decade and appear to be effective in preventing PEP. The frontrunners among these include rectal NSAIDs, prophylactic pancreatic stenting, and aggressive hydration using a LR solution.
The strategy of combining two preventive strategies appears logical, but lacks quality evidence. So far, combining rectal NSAIDs with topical epinephrine spray or aggressive hydration has not been found to be superior to NSAIDs alone.








