Abstract
Over the last half century, radiotherapy has been established as a very effective treatment modality for solid tumours. Large numbers of patients owe their lives to this treatment; however, radiotherapy is not without a price. When applied to the pelvic organs, radiotherapy carries potential serious complications, including in the small and large bowels. This article describes the magnitude of the clinical and social problems of radiation bowel disease, presents the histopathological features, and puts these features in the clinical context of the condition. The article will not address prevention and management for radiation bowel disease nor complications outside the gastrointestinal tract.
INTRODUCTION
A crucial step in the proper management of a patient with any disease is the consideration of therapy risks against its benefits. In cancer patients, clinicians are faced with the task of weighing up the benefit of prolonged, and hopefully complication-free, survival following surgery and/or chemotherapy and/or radiotherapy versus the risks of treatment- related complications. Ionising radiation is the mainstay therapy for a host of solid malignancies of the rectum, prostate, and lower urological and female reproductive systems.
As the number of cancer survivors continues to increase, the long-term outcomes related to health and wellbeing, exemplified by those patients who develop radiation bowel disease (RBD), become a focal point in health issues; therefore, there is an urgent need for a serious evaluation of prevention and management of RBD. More people with pelvic tumours are treated with radiotherapy than those with tumours at any other anatomical site and as more people live longer with cancer, the burden of RBD increases.1 It is estimated that today >3-times as many people survive cancer than 40 years ago, largely as a result of more successful multimodality therapy.1 However, up to 25% of cancer survivors report a decline in quality of life secondary to radiotherapy,2 notwithstanding the large cohort of patients who do not report the complications as they accept the condition(s) as part of cancer treatment success.
RBD results from therapy-induced damage to surrounding non-cancerous tissues, which leads to changes in the normal physiological functions of the various organs, including the small and large bowel. Treating the pelvis with radiotherapy renders the bowel at risk of radiation-induced injury, which is a condition known by the recently coined term pelvic radiation disease.1,3,4 The term denotes conditions such as radiation enteritis, radiation proctitis, and radiation cystitis.5 This article will be confined to RBD.
The initial stages of RBD involve damage to the epithelial tissue, a process that triggers an inflammatory response. For those patients who go on to develop RBD, this process will be followed by progressive ischaemia and fibrosis. The radiation damage to healthy tissue around the tumour can be responsible for treatment interruption.
A questionnaire investigating the opinion of clinical oncologists in the UK showed that most believe that RBD is an under-recognised and inadequately managed serious problem.6 Indeed, one study estimated that the annual incidence of patients adversely affected by RBD is greater than the number of patients diagnosed with Crohn’s disease.7 Several studies have shown significant complications in patients with pelvic tumours treated with surgery alone or surgery combined with either preoperative short or long-course, or postoperative, radiotherapy.8-10
As for the frequency of RBD, one study showed that 9 out of 10 patients who received pelvic radiotherapy experienced a chronic change to their bowel habits, with 5 out of 10 reporting a significant change to their quality of life.11
Not all patients who receive radiotherapy directed at tumours within the pelvis develop RBD. The reason for this is unclear; however, evidence suggests it may be a multifactorial process involving patient-related and treatment-related factors.
THE CLINICAL PRESENTATION
There is a huge range of clinical presentations of RBD owing to numerous influential variables, such as timing of radiotherapy, site of tissue damage, severity of tissue damage, side effects of medications, coexisting medical conditions, and psychological issues. The clinical presentations can be crudely classified into three phases: acute, chronic, and delayed (latent).12
Within these groups, the symptoms of RBD may manifest as a result of direct damage to pelvic structures or as secondary phenomena triggered by the radiotherapy. These phenomena include small bowel bacterial overgrowth, bile salt malabsorption, malabsorption of lactose, and similar fermentable sugars.13
The Acute Phase
Acute RBD is defined as an acute inflammatory reaction to radiation treatment that can occur during, immediately after, or within the first 3 months of radiotherapy. RBD occurs in 60–80% of patients treated with abdominal or pelvic radiotherapy and is a major risk factor for modification of the planned treatment regimen; such changes can have ramifications on local tumour control.14 Common symptoms of RBD include nausea, bloating, diarrhoea, tenesmus, abdominal cramps, urinary urgency, mucus discharge, faecal urgency, loss of appetite, and bleeding. Such non-specific symptoms are usually self-limited within 3 months but can overlap with other conditions, such as infection, which need to be excluded. Bleeding occurs in ≤50% of patients.4
Symptoms of acute RBD most commonly manifest in the second week post-radiotherapy and peak in Week 4 or 5 and resolve within 2–6 months.13 Importantly, the occurrence of acute RBD does not necessarily increase the risk of developing chronic RBD later on and patients can be reassured that resolution of symptoms generally occurs with cessation of radiotherapy.15
The Chronic Phase
Chronic RBD is a progressive condition and major cause of morbidity for cancer survivors. Symptoms of chronic RBD begin to develop after a period of 6 months to 3 years post treatment, but can occur up to three decades following treatment. Clinically, the signs of chronic RBD are symptoms of bowel dysmotility, such as urgency incontinence, change in bowel habits, and malabsorption.14 In fact, when treating rectal cancer with radiation, it has been estimated that the majority of patients will present with faecal incontinence.16
Vascular telangiectasia often leads to bleeding in the chronic phase. The timing of radiotherapy in relation to symptom manifestation is key to raising clinical suspicion and providing tailored support for RBD. Patients who experience long-standing chronic RBD can also experience sudden complications, such as bowel obstruction due to stricture formation, adhesions, fissures, severe bleeding, and perforation. Surgeons should be alert to the fact that RBD may be the cause of acute or subacute small bowel obstruction.
The Latent Phase
The third stage of the clinical pathological presentation of RBD is rare but well recognised. Latent-phase symptoms are in fact those of secondary malignancies, which can arise within or outside of the irradiation field years or decades after the initial radiotherapy treatment. Radiotherapy used to treat the first malignancy can induce minor alterations to the nuclear DNA that predispose the cell to novel DNA mutations, carcinogenesis, and teratogenesis.12 One study aimed to explain the association between radiotherapy and secondary malignancies by looking at the genetic profile of the non-cancerous mucosa. The researchers found aneuploidy in some cases that showed subtle epithelial nuclear changes in the irradiated field.17
Clinicians should be suspicious of a new tumour in any patient who has received pelvic radiotherapy and has new onset symptoms of cancer, such as rectal bleeding. Furthermore, although the risk of secondary malignancies after pelvic radiotherapy is modestly increased compared to the overall population, patients should be informed about the risk. However, some experts regard the latent phase as a complication and not necessarily a phase.
PREDISPOSING FACTORS
Predisposing factors can be broadly divided into host factors and therapy factors.12 Host factors include diabetes, atherosclerosis, hypertension, and smoking, which are all factors that accelerate the process, particularly late-phase disease, which starts as a progressive vascular phase and ends with the fibrotic phase.
The therapeutic factors that are associated with a high risk of developing RBD include high-dose radiation; a large irradiation field; timing, as postoperative radiation is more toxic than preoperative radiation; concomitant chemotherapy; and prior abdominal surgery, which leads to entrapment of the small intestine in the pelvis with similar effect of adhesion and prolapse of abdominal organs into the pelvis.1,12
THE PATHOPHYSIOLOGY OF RADIATION BOWEL DISEASE
Cells exposed to ionising radiation experience oxidative stress injuries. The damage is widespread; however, the principal subcellular target is the nuclear DNA.1
Both direct and indirect mechanisms inhibit DNA from fulfilling its function as a template for DNA transcription. The nuclear chromatin is directly targeted, causing DNA damage through the generation of inter and intra-strand cross-linkages, breaks, and mutations. The plasma membrane is directly affected, as radiotherapy disrupts the rigidity of the phospholipid bilayer and electric gradient. These types of injuries challenge the integrity of the cell. Indirect damage develops secondary to the formation of free radicals from the ionisation of water molecules.12 While the radiation damage is in progress, at the same time, intricate and co-ordinated DNA repair mechanisms have evolved to fix damage induced by ionising radiation, which includes strand breaks and replication errors.18 At low levels of radiation, repair mechanisms in the cell can resolve injuries, such as double-strand breaks. With increasing amounts of radiation, the damage inflicted overwhelms these systems and either the cell enters programmed cell death (apoptosis) or mitosis is inhibited. The amount of ionising radiation required to inflict cell inactivation and cell death varies between each tumour and its surrounding tissues.1,18
A further variable that influences a cell’s response to radiotherapy is whether adjuvant chemotherapy is included in therapy. Concomitant chemotherapy often leads to delay or prevention of the reparative process, thus aggravating the disease. The timing of radiotherapy in relation to chemotherapy is an essential consideration.19
The damaging effect of radiotherapy is most potent against tissues with high cellular turnover,3 making it ideal for treating rapidly proliferating tumour cells. This is because the potential cell injury is dependent not only on the cellular repair processes but also the stage of the cell cycle that the cell is in.
The following simplified schedule shows the relation between primary tissue type damage, phase of RBD, and the timescale of RBD symptoms:
- Acute proctitis: epithelial phase: 0–4 weeks.
- Acute enteritis: epithelial phase: 0–4 weeks.
- Rectal bleeding: vascular phase: 4–12 months.
- Anal or perianal pain: stromal phase: 6–9 months.
- Chronic abscess: stromal phase: 9–15 months.
- Fistula: stromal phase: 18–24 months.
- Stricture or malabsorption: stromal phase: 2–20 years.
- Rectal malignancy: latent epithelial phase: 5–30 years.
Certain stages within the cell cycle optimise the opportunity to repair damage. For example, ionising radiation damage results in cell cycle arrest and initiation of a temporary cell cycle checkpoint. This aims to provide time to conduct repairs. A crucial protein in the checkpoint machinery is the tumour suppressor p53. Highly proliferative cells, such as those residing in the crypt epithelium of the bowel, are frequently in the more radiosensitive G2–M phases of the cell cycle.19 Crypt cell death results in insufficient renewal of the villous epithelium. The mucosa and lamina propria become inflamed and the mucosal barrier breaks down. In comparison, slowly dividing tissues, such as those in vascular or fibrous tissue, spend more time in the less radiosensitive G1 and S phases and damage to these tissues is usually not responsible for acute clinical presentations.20
Impaired ano-rectal functionality is an important problem in RBD. Maintenance of faecal continence is regulated by the tonic contractions of the internal and external anal sphincters. The former is a smooth muscle supplied by intrinsic myenteric innervation and has the primary role of maintaining a tonic contraction and, thus, continence while at rest. By contrast, the external sphincter is composed of striated muscle and is innervated by an extrinsic supply. The internal and external sphincters work together to provide an effective seal to solids, liquids, and flatus. The ano-rectum has a rich nervous supply, which includes pain, temperature, and touch sensory components, each of which aid the maintenance of continence through the ability to differentiate between solids and flatus. Impaired anal functioning can result from damage to the nerves of the pelvis, including the pudendal nerve, the lumbo-sacral plexus, and the myenteric plexus. The external anal sphincter is relatively radio-resistant and it is postulated that faecal incontinence is strongly influenced by nerve damage. Case reports have demonstrated that damage to the pudendal nerve may lead to morphological changes in the muscle. Some case reports have proposed that injury to the lumbo-sacral plexus can indirectly affect the external anal sphincter by causing perianal anaesthesia.20
As chemoradiotherapy or radiotherapy alone often accompanies surgery in the treatment of rectal cancer, such a combination adds to the physiological disruption of normal ano-rectal function, and this needs to be factored in and communicated to the patient when such a therapy is indicated.21,22
MICROSCOPIC CHANGES TO THE BOWEL MUCOSA
An appreciation of the radiation-induced microscopic changes observed in patients with RBD provides insights into understanding the clinical symptoms, stages of the disease, and how best to manage the condition. The epithelial cells within the bowel wall, particularly those in the small bowel, have a high turnover rate, which renders them vulnerable to ionising radiation. There is a fine balance between the dose tolerated by the epithelium and the dose that destroys the neoplasm.
Histologically, the damage inflicted on surrounding healthy tissues has characteristic appearances. There are three main histological phases depending on the tissue type that is predominantly affected. The epithelial phase generally correlates with acute-phase clinical symptoms, with vascular and then stromal changes commencing several weeks later.22 Later on, the fibrotic phase complicates the picture.
In the epithelial phase, damage to the epithelium, seen as sloughing of epithelial cells into the crypt lumina, can be observed within 8 hours of exposure to ionising radiation.1 The characteristic acute-phase histological changes are epithelial meganucleosis and significant eosinophilic infiltrate with formation of eosinophilic microabscesses (Figure 1). Caution and experience are required to interpret these morphological changes because they can resemble dysplasia. Nuclear and cytoplasmic early-phase changes are usually reversible.23 Mitosis is inhibited, which prevents epithelial regrowth and causes epithelial denudation. Importantly, during the acute phase, the vasculature appears normal.23 The vascular phase follows the epithelial phase, which is characterised by telangiectasia of capillaries and post-capillary venules, fibrin deposition, subendothelial oedema, and platelet thrombi formation that can cause rectal bleeding.12 Ultimately, there is significant narrowing of the vascular lumina, which leads to ischaemia and fibrosis (Figure 2). Macroscopically, these microscopic changes correlate with a pale, non-compliant bowel wall with telangiectasia.15
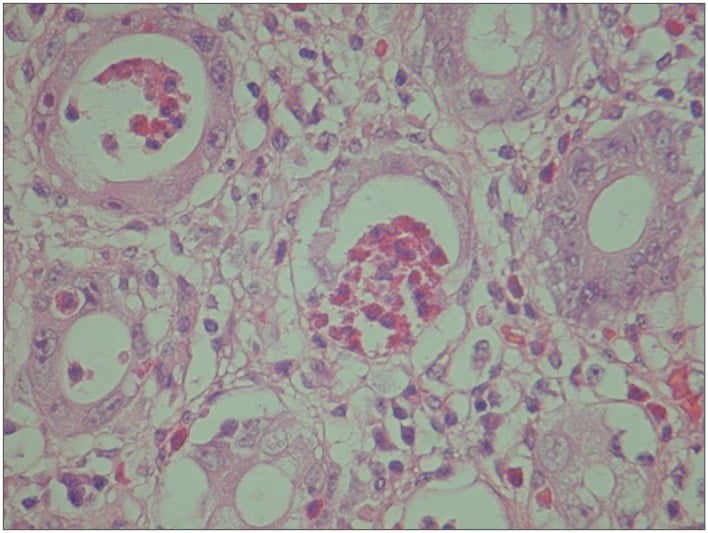
Figure 1: Eosinophilic crypt abscess with meganucleosis characteristics of an acute-phase reaction.
The nuclear abnormality should not be confused with dysplasia.
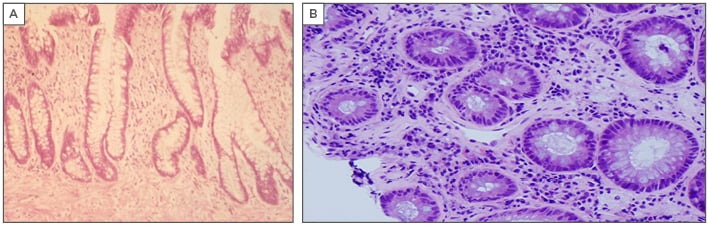
Figure 2: A) Crypt distortion and fibrosis of the lamina propria in a chronic-phase reaction mimicking chronic inflammatory bowel disease. B) Vascular telangiectasia in a chronic-phase reaction, a cause of frequent rectal bleeding.
The reversibility of the vascular phase morphological changes is unclear; however, the stromal phase, which includes mesenchymal and stromal fibrosis, is irreversible.23,24 The third phase is the fibrotic mesenchymal phase, which is a progressive phenomenon that follows and is caused by vascular damage. Fibrosis of the lamina propria leads to crypt distortion.
Despite these distinctions, the bowel has a limited array of modifications in response to damage. In fact, under a microscope the quiescent phase of inflammatory bowel disease looks the same as chronic RBD. Since chronic RBD can take months if not years to develop, it is quite possible that RBD is overlooked as a differential diagnosis and the histopathologist could remain oblivious to the patient’s history of irradiation. Relevant clinical information is therefore essential for the histopathologist; as they work through numerous rectal biopsies labelled with minimal clinical information, the biopsy from the patient with chronic RBD could be mistaken for chronic inflammatory bowel disease.25
Importantly, a study profiling the time patterns of histological mucosal changes in relation to the clinical manifestation of RBD indicated that they do not always coincide.26 Microscopic evidence of inflammation in rectal biopsies precedes the onset of symptoms. Thus, pathological changes do not always cause the symptoms, but it is the disruption to normal physiological processes that results in the symptoms such as diarrhoea and incontinence. These findings suggest that pre-emptive, prophylactic treatment to prevent RBD may be a prudent way to tackle the condition.26








