Abstract
Self-expanding metal stents (SEMS) have been established beyond doubt as an effective tool in the palliative management of malignant gastrointestinal tract strictures. The advent of fully covered retrievable SEMS has allowed its use in benign oesophageal strictures and gastric outlet obstruction, which are traditionally treated with balloon or bougie dilation. Although balloon and bougie dilations are effective, strictures may be refractory, requiring repeated sessions of dilation or complex surgeries. Endoluminal stenting spares the patient from complex surgical procedures and their associated complications. Here, the authors present four cases wherein fully covered SEMS were used as an effective therapy for the restoration of the gastrointestinal lumen in non-malignant conditions.
Key Points
1. The use of endoluminal stents means that healthcare professionals can treat patients with certain conditions such as anastomotic site leaks, strictures, and fistulas without complex surgical intervention.2. The authors discuss the use of stents in four patients who presented with different benign conditions with failed prior endotherapy.
3. While there are advantages and disadvantages to specific stents, and they should be chosen depending on the condition, the clinical experience with self-expanding metal stent is better than self-expanding plastic stents and biodegradable stents.
INTRODUCTION
Self-expanding metal stents (SEMS) have been established beyond doubt as an effective tool in the palliative management of malignant gastrointestinal (GI) tract strictures. Partially covered and uncovered stents are being frequently used in the case of oesophageal, gastric, and colonic malignancies, allowing for the endoscopic restoration of GI tract patency. The advent of fully covered retrievable SEMS has allowed its use in benign oesophageal strictures and gastric outlet obstruction (GOO), which are traditionally treated with balloon or bougie dilation. Although these dilations are effective, strictures may be refractory, requiring repeated dilations or complex surgeries such as gastric pull up, colonic transposition, and gastrojejunostomy. Endoluminal stenting spares the patient from complex surgical procedures and their associated complications, including anastomotic site leaks, strictures, and fistulas. Here, the authors present four unusual cases encountered in their clinical practice wherein fully covered SEMS were used as an effective therapy for the restoration of the GI lumen in non-malignant conditions.
CASE 1
A 70-year-old male presented to the hospital with a cervical fracture following a road traffic accident. The patient was subjected to cervical decompression and fusion. Nearly 2 weeks later, the patient developed an upper oesophageal leak that was secondary to the implant eroding through the posterior oesophageal wall (Figure 1A). The attempted closure of the leak with endoscopic clip placement and surgical correction, which involved a musculoskeletal flap placement and suturing of the oesophageal defect, failed. The patient was placed in an intensive care unit and underwent endotracheal intubation. Feeding was continued through a percutaneous endoscopic gastrostomy tube. A 10 cm long cervical SEMS (Niti-S™ Stent, Taewoong Medical, Seoul, South Korea) was placed across the defect under endoscopic and fluoroscopic guidance (Figure 1B). This SEMS had a 7 mm long proximal flange and a lumen diameter of 18 mm. The stent remained in situ for 8 weeks. Post-SEMS removal endoscopy and barium swallow showed no evidence of a leak; the patient was then started on oral feeds. The patient eventually ambulated and they were discharged without recurrence of leak.
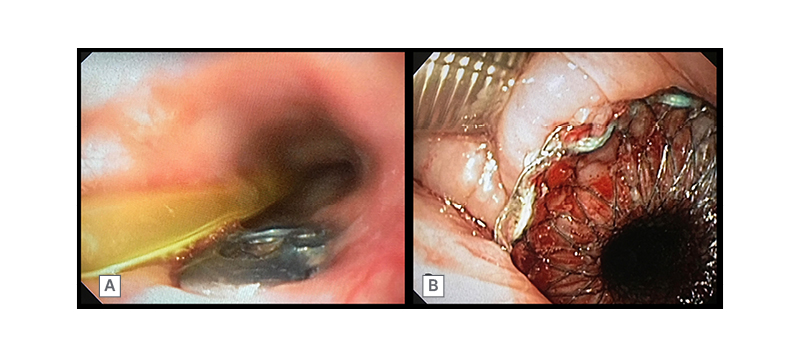
Figure 1: A 70-year-old male with cervical spine implant eroding through the posterior oesophageal wall (A). Cervical oesophageal SEMS in situ (B)
CASE 2
A 70-year-old male presented with recurrent episodes of dysphagia and regurgitation. An endoscopic evaluation revealed an epiphrenic diverticulum at the lower oesophagus (Figure 2A) and manometric evaluation revealed an underlying Type II achalasia cardia. Surgical correction with Heller’s myotomy and diverticulectomy failed to provide symptomatic relief. The patient presented with recurrent episodes of acute onset dysphagia; endoscopy would reveal food bolus impaction in the diverticulum, prompting endoscopic foreign body removal. On barium swallow, barium would initially fill up the diverticulum, causing compression, kinking, and the narrowing of the infra-diverticular oesophagus. Repeated balloon dilation of the infra-diverticular oesophageal segment did not benefit the patient. Repeat surgery was deemed too difficult because of significant comorbidities and the failure of the index surgery to provide symptomatic relief. This prompted an innovative endoscopic intervention: a padlock clip was applied at the apex of the diverticulum, shrinking it significantly, and a 10 cm long, fully covered anti-migratory oesophageal stent (Niti-S Beta™ Stent, Taewoong Medical, Seoul, South Korea) was placed, covering the diverticular region. Repeat barium studies showed a smooth flow of barium through the oesophagus into the stomach. Although stent removal was planned to take place after 3 months, the stent had to be removed prematurely at 2 months after it migrated into the stomach. Post-stent removal (Figure 2B), the patient remained asymptomatic without any further episodes of food bolus impaction and gained 10 kg of weight.
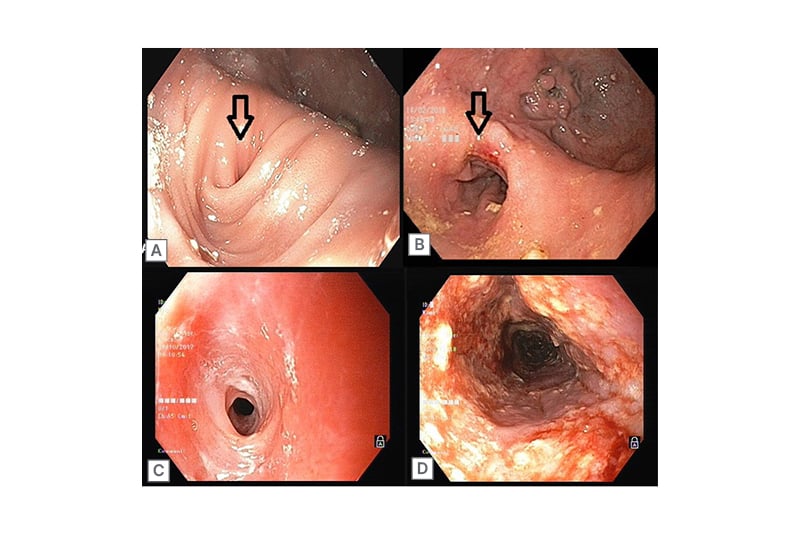
Figure 2: A 70-year-old male with an epiphrenic devericulum with infradiverticular esophageal stricture (A) and post-stent removal (B); and a 45-year-old female with a congenital oesophageal stricture (C) and post-stent removal (D).
CASE 3
A 45-year-old female presented with a 3 month history of dysphagia for solids. Endoscopy showed a long segment stricture involving the mid-oesophagus with fragile mucosa (Figure 2C). The patient denied any history of caustic upper GI injury or radiation. Oesophageal biopsies were done to rule out eosinophilic oesophagitis. Multiple sessions of bougie dilations failed to provide results. A diagnosis of congenital oesophageal stenosis (COS) was considered, and a 10 cm long, fully covered SEMS (SX-ELLA Stent, ELLA-CS, Hradec Králové, Czechia) was placed across the stricture. The stent remained in situ for 2 months and, post-removal, the patient was relieved of dysphagia. Repeat endoscopy showed a restored lumen diameter, allowing for the free passage of an adult endoscope (Figure 2D). No recurrence of symptoms have been reported over 3 years of follow-up.
CASE 4
A 56-year-old male with no comorbid illness presented with a history of accidental ingestion of an unidentified volume of unlabelled floor cleaning agent. On admission, their main complaints were retrosternal and upper abdominal burning. Chest and abdomen X-rays showed no evidence of perforation. An upper GI endoscopy was performed the next day, which showed Zargar Grade IIA and IIB injuries to the oesophagus and the stomach, respectively. The patient was treated initially with intravenous fluids and proton pump inhibitors. Oral feeds were gradually introduced, after which the patient was discharged on oral proton pump inhibitor therapy. The patient presented again 25 days later with complaints of recurrent vomiting and post-prandial upper abdominal distention. Upper GI endoscopy showed scarring of gastric mucosa in the fundus and the body, with a stenosed, eccentrically located pyloric opening (Figure 3B). The antropyloric stricture was subjected to endoscopic balloon dilation (CRE™ Balloon 12–13.5–15 mm, Boston Scientific, Marlborough, Massachusetts, USA), which was repeated three times in the following 3 months to prevent recurrence of the stricture. As the stricture was refractory to multiple sessions of balloon dilation, the option of surgery versus SEMS placement was discussed. Consequently, a 10 cm long, fully covered SEMS (Niti-S™ Stent, Taewoong Medical, Seoul, South Korea) was placed across the stricture (Figure 3A). The patient remained asymptomatic and gained approximately 10 kg of weight post-procedure. The pyloric SEMS was removed endoscopically 3 months later (Figure 3C). At a 24-month follow-up, no stricture recurrence was observed.
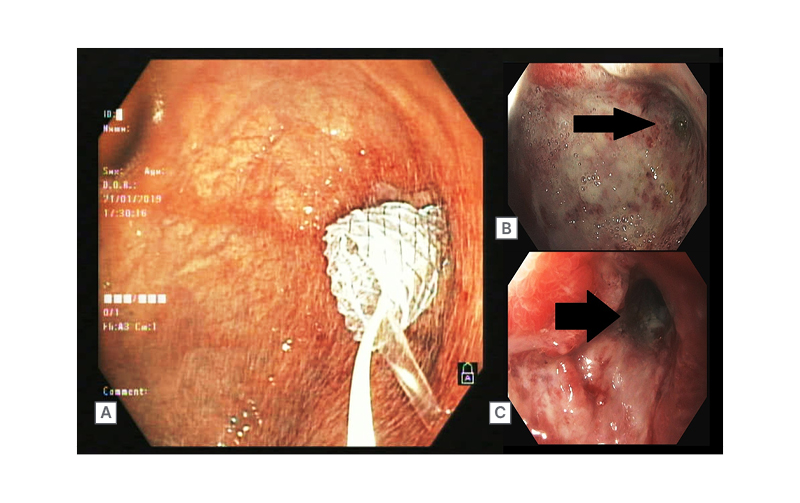
Figure 3: A 56-year-old male with corrosive gastric outlet obstruction (B), SEMS in situ (A), Post- SEMS removal (C)
DISCUSSION
Since the introduction of SEMS in the 1990s for the palliation of oesophageal malignancies, stents have evolved in design and composition, allowing for their use in treating novel indications. Several types of stents are available on the market; nonetheless, careful selection of an appropriate type of stent is vital for achieving desired results.
Self-Expanding Metal Stents
SEMS can be uncovered, partially covered, or fully covered. Uncovered (or bare-metal) stents were widely used in the palliation of inoperable oesophageal malignancies. Although effective, these had the disadvantage of dysphagia recurrence due to tumour ingrowth, which was seen in up to 36% of the patients that required repeat stenting.1 The issue of tumour ingrowth was addressed by using fully covered metal stents, which had a polymer coating around the metal frame that prevented ingrowth. However, fully covered stents presented a unique problem: stent migration.2 Currently, partially covered stents, which are covered in the middle and have uncovered portions at both the ends, are used to balance the risk of migration and tumour overgrowth. In cases of benign strictures, only fully covered SEMS can be used as tissue ingrowth makes stent removal more difficult. The risk of migration can be addressed by using stents with flared ends and fixing stents using haemoclips,3 over-the-scope clips,4 or endoscopic suturing.5
SEMS have also been modified according to the intended location of their use. In cases where the lesions are in proximity to the upper oesophageal sphincter, foreign body sensation and injury preclude the use of regular SEMS. A special modification of the SEMS with a short and narrow funnel in the proximal end has allowed for its use in lesions of the cervical oesophagus.6 In cases involving the oesophagogastric junction, the placement of SEMS across the lower oesophageal sphincter complex has been associated with severe gastro-oesophageal reflux; however, SEMS with anti-reflux valves have been shown to reduce reflux symptoms.7
Self-Expanding Plastic and Biodegradable Stents
Although fully covered SEMS have made the non-surgical treatment of refractory benign strictures possible, they are not devoid of drawbacks. Complications such as the recurrence of the stricture, hyperplastic tissue reaction at the ends, perforation at the edges, and migration present difficulties.8 Plastic stents were introduced, which were proposed to have several advantages including low cost, minimal tissue reaction, and easy placement and removal. Several studies have shown good results with self-expanding plastic stents (SEPS).9,10 Biodegradable stents (BDS), which are made of biodegradable material that gradually disintegrates over 11–12 weeks post-stent placement, are a newer addition to the armamentarium. The radial force of these stents is maintained over 5–6 weeks, avoiding the need for reinterventions for stent removal.11
Analysis
A meta analysis by Fuccio et al.12 compared the results of 18 studies that used SEPS, SEMS, and BDS in treating refractory benign oesophageal strictures. Overall, the pooled clinical success rate was 40.5%. The use of SEPS and SEMS did not result in significantly higher success rates than with BDS (46.2% versus 40.1% versus 32.9%, respectively). The migration rate for SEPS and SEMS were reported as not being significantly higher than for BDS (33.3% versus 31.5% versus 15.3%, respectively). Another prospective, multicentre study13 comparing the three different stent types concluded that BDS or a fully covered SEMS may lead to long-term relief of dysphagia in 30% and 40% of patients, respectively. The use of SEPS was the least preferable option due to frequent stent migrations and the need for reinterventions.
Although each stent category has its advantages and drawbacks, experience with fully covered SEMS is generally better than with SEPS and BDS. Moreover, the design of stents and our experience with novel stents may improve with time. However, SEMS placement, although capable of marvellous clinical outcomes, is not without complications. These complications can be early, occurring within 4 weeks of stent placement, or late, occurring after 4 weeks.14 Early complications include foreign body sensation, pain, gastro-oesophageal reflux, migration, bleeding, and perforation. Late complications include stent migration, stent block due to food impaction, tumour ingrowth, and tumour overgrowth.
In a retrospective analysis by Na et al.,15 complications related to stent placement were found in nearly 40.3% of patients. The complication rate was lower, at 32.6% in cases using the latest generation stents. Chest pain following stent placement was seen in 6.7% of patients, but the majority responded to analgesic therapy. Few patients required stent removal due to intractable pain that did not respond to analgesics. The pain was more common in stents made of stainless steel rather than those made of nitinol, and in patients who had undergone radiation therapy before stent placement. Stent migration was seen in 10.9% of the patients and was managed either by stent repositioning or stent replacement. Bleeding after stenting was seen in 1.9% of patients; in most, the bleeding stopped spontaneously within 48 hours. Only one patient required adrenaline injection and another required embolisation after stent removal. Membrane degradation, membrane separation, and incomplete expansion of the stent are other less common complications. Nonetheless, further development in stent design and materials may decrease the complication rates and increase clinical success in the near future.
In the first case mentioned, the patient presented with a post-traumatic cervical spine injury. Anterior cervical spine surgery with implant fixation is a commonly performed surgery for the management of degenerative or post-traumatic cervical injury. Although rare, oesophageal perforation occurring post-anterior cervical spine surgery can cause significant morbidity.16 Factors thought to contribute include the suboptimal placement of the implant and implant failure or breakage, exposing sharp edges that can erode through the oesophagus. The mass effect of the implant on the oesophageal wall, and ischaemia secondary to pressure while swallowing have also been proposed as possible causative factors.17
Whatever the cause, the resultant perforation is usually managed with implant removal whenever feasible. Surgical correction with primary closure and a sternocleidomastoid flap is considered the gold standard for the treatment of such cases.18 In this case, as the flap placement and primary closure failed to plug the leak, an out-of-the-box solution was used wherein the SEMS effectively closed the oesophageal leak while retaining the cervical implant for effective healing of the fracture. The choice of an appropriate stent was crucial. As per conventional wisdom, stent placement within 1–2 cm of the upper oesophageal sphincter is contraindicated as it is associated with a high risk of perforation, aspiration, tracheal compression, and foreign body sensation.19 However, the introduction of the cervical stent has made this task possible. The stent had a narrow proximal flare, resulting in less pressure in the proximal oesophagus, thereby minimising injury. The authors chose a fully covered stent as this was a benign case with stent removal planned after 10–12 weeks. Although distal migration of the stent was another concern, the use of clips was not possible in this case due to the proximity of the stent to the upper oesophageal sphincter.
In the second case, a symptomatic oesophageal diverticulum recurred post-surgical correction. As a repeat surgery was considered risky due to comorbid conditions, SEMS was utilised. Oesophageal diverticula are rare disorders presenting with dysphagia, chest pain, and regurgitation. Depending on their location, they can be categorised as epiphrenic, Zenker’s, or Rokitansky diverticula. Epiphrenic diverticula are pseudodiverticula of the pulsion type that are located in the distal oesophagus. Oesophageal motility disorders and congenital weakness in the oesophageal wall have been thought to be pathological factors, and 75–100% of patients with an epiphrenic diverticulum have an underlying primary oesophageal motility disorder.20 Many patients may remain asymptomatic and do not require interventions. Symptomatic patients may have dysphagia and/or regurgitation, and some may present with respiratory symptoms like aspiration, asthma, etc.21,22 All symptomatic patients require intervention.
Traditionally, surgical resection of the diverticulum has been advised either with a transthoracic approach ,or by laparoscopy. Myotomy and fundoplication are usually performed together alongside the resection when an underlying motility disorder is suspected.23 Endoscopic per-oral endoscopic myotomy with septotomy for the treatment of distal oesophageal diverticula was also considered. While these approaches have good long-term results, leaks are common, which can cause significant morbidity, so these may be a difficult option in high-risk patients. Only a few cases have been reported in the literature where a surgically unfit patient has been treated with SEMS placement.24
The Niti-S Beta™ Stent (Taewoong Medical, Seoul, South Korea) used in the present case is a fully covered oesophageal stent that was designed for the treatment of suture line leaks occurring after bariatric surgeries. The stent has two bumps over the body that prevent migration by increasing the radial pressure. As the oesophageal narrowing in this case was focal with proximal oesophageal dilation, the risk of stent migration was high. A Beta stent was chosen to minimise the risk of migration; additional stent fixation or suturing was not necessary. A study that used Beta oesophageal stents for anastomotic leaks and following a total or proximal gastrectomy reported a migration rate of 7.1%, without any additional stent-fixing procedures. The stent migration rate was 13% for conventional stents with clips and 17% for suture fixation.25
On the other hand, another retrospective study investigating the use of Beta oesophageal stents in staple line leaks showed a migration rate of 32%, with no significant decrease in the stent migration rates.26 In this case, despite using an anti-migratory stent, the stent migrated distally after 8 weeks. Although this duration was sufficient to dilate the stricture in the present case, an additional application of haemoclip or an over-the-scope clip could be considered to prevent early migration.
Equally rare is the non-surgical management of an adult-onset presentation of COS, a rare disorder that usually presents in infancy or childhood and manifests with progressive dysphagia and vomiting.27 Three forms of the disease are known to exist: membranous diaphragm; hypertrophy and fibrosis of the submucosa and muscularis propria; and tracheobronchial remnants like cartridges and glands in the oesophageal wall. As rare as its incidence, it is rarer for the disease to go unnoticed till adulthood. Nevertheless, many cases of mild stenosis going unnoticed and being diagnosed in adulthood have been reported in the literature.28,29 Classically labelled as ‘slow eaters’, a modification of diet in cases with mild dysphagia may lead to delayed recognition of the problem. Once diagnosed, the treatments that are usually considered include dilation and surgery.
Many case reports have reported success in treating adult-onset cases with bougie dilations.29,30 A systemic review by Terui et al.31 concluded that both balloon and bougie dilations are effective forms of treatment for COS. Dilation was less effective in the tracheobronchial subtype of COS, and surgery has been recommended in those instances. The success rate in dilation with or without case selection (endoscopic ultrasound to exclude the tracheobronchial subtype of COS) was reported to be 89.7% and 28.9%, respectively. The rate of perforation with or without case selection was 7.4% and 23.9%, respectively. In this case, repeated dilation failed. Although the next logical mode of therapy would be surgery, SEMS placement was an innovative, non-surgical therapy that was successful in alleviating the symptoms. The literature search did not return any clinical trials studying the use of SEMS in COS. Although SEPS and BDS could have been used in this case, the authors considered the metal stent as they had more personal experience using SEMS compared with SEPS and BDS. Stent migration was not considered to be a challenge in this case due to the length of the stricture, so stent fixation was not considered.
In the final case of post-corrosive injury with GOO, SEMS was used successfully to restore the patency of the GI tract. Corrosive injury of the upper GI tract remains a frequent problem with high post-injury morbidity. Alkali ingestion affects the oesophagus, while gastric injuries are marked with acid burns; the squamous epithelium of the oesophagus is more resistant to acid penetration, while the free gastric acid buffers the alkaline agents, limiting injury.32-34 GOO usually manifests 3–4 weeks post-injury, but can be as early as 1 week, as per a few case reports.35,36 Endoscopic balloon dilation has been proved to be an effective intervention in patients with corrosive GOO. However, unlike in ulcer-related GOO, the recurrence of the stricture is common in caustic GOO, and is seen in up to two-thirds of patients.37,38 SEMS placement can be used as an alternative therapy in such patients.
Manta et al.39 treated three patients with refractory corrosive antral stenosis with SEMS placement, and Choi et al.40 treated 22 patients with benign antropyloric stenosis with SEMS. Despite stent migration being a major concern, most patients with late SEMS migration remained asymptomatic. SEPS and BDS are predominantly used for benign oesophageal lesions, and there is scant information in the literature regarding their use in either benign or malignant GOO. The authors used a fully covered SEMS with a wide flare; the duodenal bend also acts as an anchor, decreasing the chances of migration, so no stent fixation methods were employed. Another unique problem reported with the use of SEMS in treating caustic strictures is the extensive granulation tissue proliferation at the ends of SEMS, leading to ingrowth and membrane disintegration. These factors can interfere with stent removal and may require the use of argon plasma coagulation to separate the stent from the granulation tissue.39 SEMS placement may be an effective alternative to surgery in corrosive GOO, but more studies are needed to evaluate this treatment.
CONCLUSION
Benign oesophageal and gastric strictures and leaks may be managed by surgery or endoscopic therapy. In this paper, the authors outline a few complex and rare presentations of cases that were managed with innovative, mostly out-of-the-box treatment methods. SEMS placement successfully re-established the luminal patency with good clinical outcomes and minimal complications in all of these cases.







