Abstract
Background: Infliximab (IFX) trough levels measurement could partially explain mechanisms of loss in response to this drug. However, little information exists on its concentration at the mucosal level or mucosal pharmacokinetics.
Objective: The aim of this study was to investigate whether IFX could be measured within intestinal mucosa, and whether a correlation between mucosal level, serum level, and clinical response could be hypothesised.
Methods: Fifteen consecutive patients with inflammatory bowel disease receiving stable doses of IFX who underwent endoscopy were enrolled. Biopsies were taken from an affected and an unaffected area and cultured for 48 hours, and serum samples were also collected. IFX and tumour necrosis factor alpha (TNF-α) levels were measured using commercially available enzyme-linked immunosorbent assay kits.
Results: IFX levels were detected in 80% of the colonic biopsy supernatants and in 60% of the serum samples. TNF-α intestinal mucosal levels were detectable in 100% of patients, while TNF-α serum levels were detectable in 75%. Mucosal and serum levels of IFX and TNF-α did not correlate; no correlation was found between the last infusion and serum or intestinal mucosal levels. Levels of IFX were more frequently undetectable in the mucosa of patients not responding to IFX therapy.
Conclusions: Detectable levels of IFX and TNF-α can be found in intestinal mucosa. IFX mucosa levels could be useful to stratify patients into responders and non-responders to IXF therapy.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory condition characterised by an uncontrolled mucosal immune reaction.1 The external environment, the patient’s genetic makeup, the intestinal microbial flora, and the immune system are collectively involved and functionally integrated in the development of the chronic intestinal inflammatory reaction that characterises IBD (mainly Crohn’s disease [CD] and ulcerative colitis [UC]).2 Despite several interesting papers that dissect complex aspects of IBD pathogenesis, a unified theory is still unknown. Therapeutic options comprising classical therapy, immunosupressants, and biologics are not completely orientated to counteract IBD pathogenesis.
Tumour necrosis factor alpha (TNF-α) is a pivotal pro-inflammatory cytokine in the immune pathogenesis of IBD.3 It is involved in the activation of immune and non-immune cells, and released in soluble form from the transmembrane protein (mTNF-α).3,4 Infliximab (IFX), a chimeric mouse-human monoclonal antibody composed of constant regions of human immunoglobulin (Ig)G1k coupled to the variable regions of a high-affinity (Ka=1010M-1) neutralising murine anti-human TNF-α antibody, is the first example of a biological drug in IBD treatment, following discoveries in recent years regarding an immune-pathologic basis of various chronic diseases. As shown by Scallon et al.,4,5 IFX binds both soluble and mTNF-α, and activates an in vitro antibody and complement-dependent cellular cytotoxicity by its Fc portion. This causes apoptosis in Jurkat T cells in vitro, and in vivo on lamina propria mononuclear CD3+ cells in patients with chronic active CD through the activation of caspases and increase in Bax and Bak proteins.6 Further studies demonstrated its role in decreasing expression of the vascular cell adhesion molecule 1 and CD40 proteins on endothelial cells, in modulating fibroblast cell migration,7 and in increasing basic fibroblast growth factor and vascular endothelial growth factor.8
IFX can also induce cell lysis through antibody-dependent cell cytotoxicity or complement- dependent cytotoxicity, activated by cell-bound IFX.4,5 Several experimental data in vitro9,10 and in animals6,9-13 have shown that IFX acts on immune cells (i.e. lamina propria mononuclear cells,7 peripheral blood leukocytes,14,15 lymphocytes,16 and monocytes),17 but also on other intestinal cells such as epithelial18 and endothelial cells,19 fibroblasts,7 and platelets.19
However, the success of anti-TNF-α strategy in IBD treatment is demonstrated by clinical studies which have shown a clear efficacy of IFX in inducing remission and the maintenance of remission in CD and UC patients.20-23 Furthermore, IFX has been used in paediatric IBD and is part of European and national guidelines.21,24,25 The effectiveness of anti-TNF-α blockers in IBD is demonstrated by clinical trials that show a clear efficacy of adalimumab, a humanised anti-TNF-α antibody for the induction and maintenance of clinical remission in CD and UC patients.26 However, this effect is not a pure ‘class effect’, as other anti-TNF-α blockers, such as etanercept, that are efficacious in rheumathological diseases, did not show clinical efficacy in IBD. As it was utilised in clinical trials, IFX displayed good efficacy and safety profile data in adult and paediatric patients affected by moderate-to-severe CD and UC.20-23,27
Despite this, clinical trials for other therapeutic approaches in IBD and clinical use have shown a primary failure or a subsequent lack of response to IFX in 20–50% of cases.28 Several mechanisms have been proposed to explain this:
- Reduced serum levels of IFX (also known as trough levels when measured directly before the next infusion) may be a result of suspected higher utilisation, loss, or unspecified metabolic reasons. Low trough levels of IFX are associated with a worse clinical outcome.28-30 This could be indirectly verified with different therapeutic strategies, including an increase in IFX dose or in the number of administrations within a set time-span28,31-33
- Formation of antibodies to IFX (ATI), which may affect serum IFX levels as well as interact with the binding capacity of IFX to circulating or mTNF-α. The higher the level of ATI, the higher the chance of finding low levels of circulating IFX or a lack of response to IFX.29,33,34 ATI are found in up to 25% of patients who lack detectable serum trough levels of IFX28,33,35
- Changes in the biological signature of the disease due to the activation of other key inflammatory molecules, independent of or only partially dependent on TNF-α pathway33,36
A role may also be played by other autoantibodies, such as an antinuclear antibody, double-stranded DNA, single-stranded DNA, anti-histone antibodies, and other influencing factors which are yet to be investigated. Notwithstanding these observations, it should be recognised that little data exists on the pharmacokinetics or pharmacodynamics of IFX. Because of its long half-life and slow clearance rate, IFX remains detectable in serum for at least 8 weeks after a single infusion of 5 mg/kg.37,38 Despite several direct and indirect studies that showed IFX with measurable effects at mucosal levels, only a single paper has shown that the drug could be found at the intestinal mucosal level and that it acts through direct contact of IFX with intestinal cells.39 Excretion paths of IFX are also not yet completely understood. Similar limits apply for animal models of colitis, as demonstrated by our recent paper.40
The finding that a local injection of IFX is effective in sustaining fistula closure in patients with perianal CD41 raised new attention to direct effects of IFX at intestinal mucosal levels. This observation has recently been confirmed using adalimumab,42 supporting the hypothesis that anti-TNF-α antibodies are able to act not only systemically, but also locally in IBD patients, as shown by Caprioli et al.43 who demonstrated changes in immune cells within intestinal biopsies following intravenous IFX treatment. Moreover, IFX contributes to the mucosal healing process by acting directly at the level of the intestinal mucosa, where it induces apoptosis of leukocytes, and directly sustains epithelial cell migration and proliferation, together with the induction of p44/p42 extracellular signal-regulated kinases, as a result of the neutralising effect of TNF-α.44
Clarifying these aspects correlated to the mechanisms of action of this drug, together with its pharmacokinetic aspects, could lead to new understandings of the mechanisms controlling the lack or loss of response to it. This could support the development of new drugs, perhaps more efficaciously or at least more selectively, as in the case of mesalazine which is derived from sulfasalazine or meropenem from other penicillins.45 Starting with these controversies, the purpose of this study is to explore whether measurable levels of drugs are detected at intestinal mucosal levels. This parameter will be correlated to serum levels and evaluated as a potential factor in delineating IBD patients responding or not responding to IFX.
METHODS
Patients
Informed consent was obtained for all patients. Following this, the 15 consecutive patients with moderate-to-severe IBD receiving stable maintenance doses of IFX (8 female and 7 male; 7 with CD and 8 with UC) were enrolled when admitted for routine endoscopy 3±1 weeks from the last IFX infusion. Medical history and clinical records (in particular the day and dosage of last IFX infusion) were examined. The mean age of the patients enrolled was 42.2±15 years (Table 1). At the time of endoscopy, clinical characteristics and disease activity were registered for each patient, and peripheral blood and intestinal biopsies were collected. For five of these patients, biological samples were obtained up to 1 year before starting IFX, which also required consent. These were utilised as a control for assessing the specificity of the ELISA kit (data not shown).
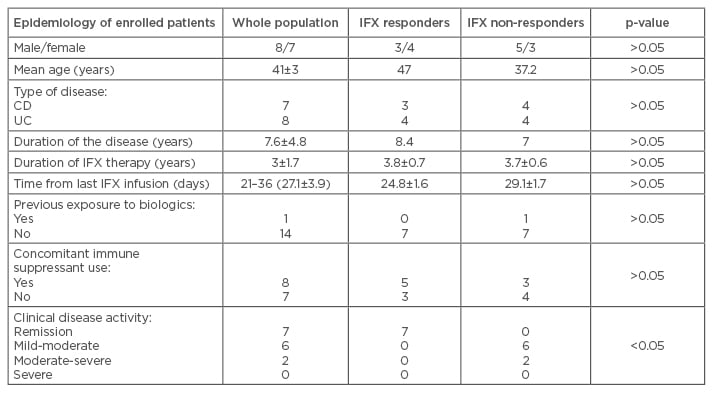
Table 1: Clinical characteristics of inflammatory bowel disease patients enrolled in the study.
IFX: infliximab; CD: Crohn’s disease; UC: ulcerative colitis.
This study received formal approval from a local ethical committee of Policlinico Universitario Agostino Gemelli, University of the Sacred Heart Rome, Rome, Italy, in 2011. Diagnosis of CD as well as UC was performed in agreement with current guidelines. Clinical disease activity was evaluated following the Harvey Bradshaw Index for CD, and the Colitis Activity Index for UC, both of which were utilised to define the activity of disease; several papers have reported a good correlation between ‘clinical judgment’ and these evaluation methods. All parameters, including the number of bowel movements, bloody diarrhoea, abdominal pain, or bloating, together with joint pain and extra-intestinal manifestations of the disease, were registered as indicated. Conversely, the clinical response to IFX was considered based on clinical judgment. In particular, persistence of symptoms, no C-reactive protein improvement, and worsening of the disease following at least 3 months of infliximab (Table 1).
Intestinal Biopsy Culture
As previously described,44 intestinal biopsies from enrolled IBD patients, treated or naïve for IFX, were taken from inflamed and uninflamed areas, washed with phosphate-buffered saline, weighed, and put in a culture for 48 hours in regular culture media (Dulbecco’s modified Eagle’s medium [D-MEM] with 20% fetal bovine serum and antibiotics/fungizone) on 30-minute polyethylene terephthalate track-etched membrane in 24 multiwell plates (VWR International, Radnor, Pennsylvania, USA). Biopsies were taken by endoscopic loop used for regular and commercially available diagnostic sampling. The weight of the samples were similar, ranging from 6–15 mg, while the median length of the samples was 3 mm. After 48 hours, supernatants from colonic specimens were collected and stored at -80°C for further analysis.43 Histological haematoxylin and eosin staining was also performed to assess the viability of the cells (data not shown).
Enzyme-Linked Immunosorbent Assay for Tumour Necrosis Factor Alpha and Infliximab
TNF-α levels within the serum and within intestinal biopsy supernatants were measured by Enzyme-Linked Immunosorbent Assay (ELISA; R&D systems, Minneapolis, Minnesota, USA), following instruction from the manufacturer. IFX levels were measured from the same samples through a commercially available TNF-α blocker-monitoring ELISA IDKmonitor® (Immundiagnostik, Bensheim, Germany), following instruction from the manufacturer. Briefly, drug levels were measured by spectrophotometry at 450, 540, or 579 nm wavelengths and estimated using a SpectraMax® Plus 384 Microplate Reader (Molecular Devices LLC., Sunnyvale, California, USA) spectrophotometer. The results are expressed as μg/mL by the programme SoftMax PRO (Molecular Devices) following interpolation.
Results from supernatants of the intestinal specimens, similar to those from murine samples in our recent paper,40 were related to the total protein concentration, and evaluated by spectrophotometry at λ of 650 nm using the Bradford colorimetric method. IFX concentrations between 0.4 and 45 μg/mL were considered to be within the measuring range of the assay, according to the manufacturer. Readings below 0.4 μg/mL were considered to be undetectable levels of IFX.
Statistics
Statistical analysis was performed using a T Test or ANOVA with Bonferroni correction/Fisher/chi test, depending on the experimental setting. Pearson correlation coefficient was used to correlate mucosal levels of IFX or TNF-α either to time or to serum levels of IFX or TNF-α. Mean, median, and standard errors and deviations were used as indicated.
RESULTS
In this study, 15 patients were enrolled (7 with CD, and 8 with UC) whose clinical characteristics are summarised in Table 1. This was a small cohort of IBD patients with typical features: young age, reasonably long exposure to IFX therapy, and concomitant use of an immune suppressant in about 50% of cases. These features are suggestive of a population with an aggressive course of the disease. As expected, between patients responding and not responding to IFX therapy, there was a statistically significant difference in disease activity index. Non-responders proved more likely to display a more active disease compared with responders.
Measurable Levels of Infliximab in Serum and Intestinal Mucosa
The commercially available kit to measure IFX in serum was successfully utilised to assess drug levels on supernatants of intestinal mucosa, and showed a good specificity. We evaluated pre and post-therapy IFX serum levels in the same patients (data not shown). IFX levels before starting the treatment were below the detection limit, while those from the same patients after IFX infusion were above the detection limit. Detectable levels of IFX in serum were found in >60% of patients, while in intestinal biopsies this percentage increased to 80%. Although a direct comparison between IFX levels from serum and intestinal biopsies cannot be conducted, serum and mucosa show opposite findings when we consider IFX levels or TNF-α levels (Figure 1). TNF-α levels in both serum and intestinal biopsy supernatants were detectable in almost 100% of the cases. No difference in IFX levels was found between inflamed and uninflamed intestinal mucosa (data not shown).
Figure 1: Levels of infliximab and tumour necrosis factor alpha in serum and intestinal mucosa.
Detectable levels of IFX have been shown in 60% and 80% of patients in serum and in intestinal biopsies, respectively (readings below 0.4 μg/mL were considered to be undetectable levels of IFX). Detectable levels of TNF-α have been shown in 100% of patients in serum and intestinal biopsies. In the present figure, values of IFX and TNF-α have been reported for serum (μg/mL) and for biopsies (μg/mg of protein).
IFX: infliximab; TNF-α: tumour necrosis factor alpha.
Poor Correlation Between Mucosal Levels of Infliximab and Tumour Necrosis Factor Alpha with Serum Levels, and Days from Last Infliximab Infusion
In order to find major determinants of IFX mucosal levels, a possible correlation with serum IFX or days from last infusion was assessed. Poor or no correlation was found between intestinal and serum levels when data from the full cohort of patients were pooled together. Similar findings were found when TNF-α was considered (data not shown). A weak correlation was also found between the serum and intestinal mucosal levels, and time from last IFX infusion. The former proved more dependent upon time from last IFX infusion than the latter.
Serum and Mucosal Levels of Tumour Necrosis Factor Alpha and Infliximab in Patients Stratified by Response to Infliximab
No significant differences were found among serum levels of TNF-α and IFX when patients were stratified based on response to IFX. However, a clear trend in accordance with data from literature was found (Figure 2A). When stratifying for detectability rate in responders or non-responders to IFX, the likelihood of measuring undetectable mucosal levels of IFX was higher in non-responders compared with responders to IFX therapy (p<0.05) (Figure 2B).
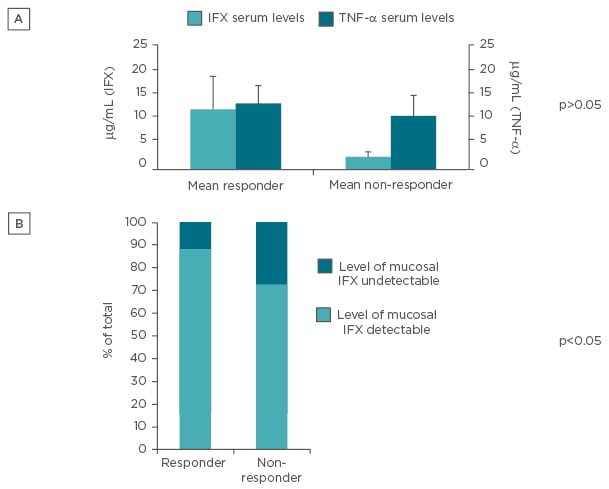
Figure 2: Levels of drug and response to it.
A) Serum IFX and TNF-α levels in patients stratified for clinical response to IFX; B) percentage of detection of IFX within intestinal mucosa stratified for clinical response to IFX.
IFX: infliximab; TNF-α: tumour necrosis factor alpha.
DISCUSSION
This study displays several new findings with important possible implications for clinical and basic science research involving mechanisms of action/loss of response of anti-TNF-α in IBD. Measurable IFX levels, using commercially available kits, could be found in serum and within intestinal mucosal supernatants obtained with traditional organ culture techniques, or even in faecal samples as shown by our recent publication in mice.40 The presence of IFX within intestinal mucosa implies a direct interaction of IFX with different cell populations within the intestinal mucosa, including both immune cells (i.e. lamina propria, dendritic cells, macrophages, and leukocytes), and non-immune cells (i.e. endothelial cells, fibroblasts, and enterocytes). This aspect, in complete agreement with the present paper, has been well presented recently in articles by Atreya et al.46 and Yarur et al.39 These demonstrated the interaction between the anti-TNF antibody and intestinal immune cells displaying mTNF-α during confocal laser endomicroscopy. We demonstrated that IFX levels could be found within intestinal mucosa of patients in stable treatment with IFX between 20 and 30 days following intravenous IFX infusion. This finding was verified for >80% of analysed samples, in contrast to 60% of serum samples.
Serum levels were collected during the same day of the endoscopy procedure and therefore could not be considered trough levels, i.e. the lowest level of a drug in the serum before the next drug infusion, which would have been registered at Day 56 from the last IFX infusion. The usual interval between two different IFX infusions is 8 weeks. This aspect is a limitation of the study. Almost all the publications dealing with IFX levels refer to trough levels. In the present paper, in which the hypothesis of the study is centred on intestinal mucosa, the time for serum sampling was dictated by the day of the colonoscopy assessment. Being a preliminary proof-of-concept and observational study, we did not expose patients to endoscopy if it was not needed for a clinical reason. For this reason, we obtained only one or two samples for each patient and were unable to produce a detailed pharmacokinetic report for each, instead we pooled data from different patients. With these limits, no direct correlation was found between mucosal and serum IFX levels. This observation suggests that the drug distribution at a peripheral level can be considered a different parameter from serum concentration, something which is probably dependent upon other factors, such as elimination rate, degradation rate, or use of a concomitant immunosuppressant, as recently shown by Hayes et al.47
IFX serum levels were weakly dependent upon the time from the last infusion. This parameter is clearly associated to time in other manuscripts. The weak correlation that we observed could depend on different kinetics in different patients, or on varying disease statuses among participants. An even lower correlation was found between time from last infusion and IFX intestinal mucosal levels, most likely suggesting that tissue levels are more stable than serum levels.
Our study does not provide definitive data on patients who did not respond fully to IFX, and so it remains a proof-of-concept study. Raw data suggests that a difference between responders and non-responders to IFX could be present due to IFX intestinal levels or TNF-α levels, rather than serum levels. However, dedicated and correctly powered studies are required to elucidate this issue. Loss of response to IFX (and to biologic agents in general) is an emerging challenge in the biological area, especially because few valid options are currently available within the therapeutic armamentarium for complicated moderate-to-severe disease. In a world ruled by clinical trials, the use of patient-specific parameters reinforces the personalised approach and helps the optimisation of the available medications, in order to prolong their use as long as possible and to save the necessity for further medical options.
If mucosal drug levels show a correlation with the loss of response to the drug, new and important implications will present themselves in order to selectively modify these levels. Peripheral drug levels could be increased, perhaps by increasing dosage and intervals, but also by designing drugs that could reach their target directly from the blood flow, for which the orocecal approach could prove promising. In a possible future trial dedicated to this project, patients affected by CD and UC should remain separated, perhaps even stratified per disease location. Furthermore, a more careful plan of endoscopy assessment should be implemented, keeping it closer to the timing of the trough level assessment.
A direct IFX effect on all the mucosal components could play a key role in the ability of IFX to induce mucosal healing in IBD, and to ensure a clinical response to it. However, the precise mechanism of action within intestinal mucosa needs further study, particularly by assessing whether IFX is bound to mTNF-α as well as soluble TNF-α. Measurement of IFX levels at intestinal mucosal levels, with or without TNF-α assessment and together with serum measurement of IFX and TNF-α, could provide new and important information about mechanisms of loss of response to IFX in IBD patients, as has been recently suggested.48 Further studies are necessary to provide more strength and clinical utility to the new findings proposed in our study.








