Abstract
Gastric gastrointestinal stromal tumour is an extremely rare condition to occur after bariatric surgery. To the authors’ knowledge, only two cases of gastric gastrointestinal stromal tumours after Roux-en-Y gastric bypass have been reported in the medical literature, both occurring in the excluded gastric remnant. Herein, the authors report the third case of gastric gastrointestinal stromal tumour post-Roux-en-Y gastric bypass, and the first case to occur in the gastric pouch, which was managed surgically by laparoscopic resection. From here, combining the observations of clinicians treating and following up patients post-bariatric surgery in an international database will be beneficial to patients, and aid in development of surveillance guidelines.
Key Points
1. Obesity is a global problem in healthcare and is growing in prevalence, with gold standard treatment being metabolic and bariatric surgery to aid weight loss.2. Most literature on gastric gastrointestinal stromal tumours Roux-en-Y gastric bypass indicates that incidence of obesity-related cancers decreases after bariatric surgery. Only two cases have been reported in the literature and this is considered extremely rare.
3. As gastrointestinal stromal tumours after bariatric surgery are so rare, there are no guidelines; therefore, the authors note that an international database on these cases would be beneficial in producing guidelines.
INTRODUCTION
Obesity is a rising global epidemic with a growing prevalence. A well-known association between obesity and increased overall mortality has been established. In addition, obesity is firmly linked to an increased risk of cancer. Metabolic and bariatric surgery is the gold standard treatment for long-term weight loss and resolution of associated comorbidities. With this in mind, the number of patients with previous history of bariatric surgery is continuously rising.
On the other hand, the association between intentional weight reduction through bariatric or metabolic surgery and reduced cancer risk remains poorly understood, with limited knowledge and conflicting data in the medical literature. Most studies report a decrease of obesity-related cancers in patients undergoing bariatric surgery. In fact, only a few cases of gastric cancer post-bariatric surgery have been reported in the medical literature, with the majority being gastric adenocarcinomas and two cases of gastric gastrointestinal stromal tumours (GIST); however, evidence is lacking concerning the link between gastric cancer and bariatric or metabolic surgery.1-3 From here, physicians caring for patients post-bariatric surgery should suspect malignancy in any patient who presents with vague epigastric symptoms, unexplained anaemia, gastrointestinal bleeding, and excessive weight loss. Herein, the authors report the third case of gastric GIST post-Roux-en-Y gastric bypass (RYGB) and the first gastric pouch GIST post-RYGB.
PATIENT INFORMATION
A 40-year-old male patient with a previous surgical history of RYGB 12 years ago, as treatment for morbid obesity with a BMI of 57.59 kg/m2 (195.00 kg; 1.84 m), and a normal initial pre-operative gastroscopy, presenting with the complaint of fatigue, melena, and de novo weight loss of 10 kg over a 2-month period. They reported that the nadir weight achieved was 125.00 kg at 2 years after their surgery, which was maintained for 3 years. This was followed by a weight regain of 30.00 kg, from which they lost the unintentional 10.00 kg, with the current weight being 145.00 kg, for a BMI of 42.82 kg/m2.
CLINICAL FINDINGS
Vital signs were stable and the physical exam was normal.
DIAGNOSTIC ASSESSMENT
Consequently, the patient was investigated by blood workup showing iron deficiency anaemia with a haemoglobin level of 7.5 g/dL (norm: 13.0–17.3 g/dL), haematocrit level of 29.6% (norm: 39.0–50.0%), iron level of 20 µg/dL (norm: 35–150 µg/dL), ferritin level of 2.85 µg/L (norm: 20.00–250.00 µg/L), vitamin B12 level of 363 pg/mL (norm: 191–800 pg/mL), and folic acid level of 5.66 ng/mL (norm: 5.00–20.00 ng/mL). This was followed by a gastroscopy (Figure 1), which showed a peri-anastomotic bleeding ulcer and a 5 cm round submucosal tumour in the lesser curvature of the gastric pouch, just proximal to the gastro-jejunal anastomosis. In addition, the endoscopist noted a large anastomosis and a long blind loop. Biopsies were taken from the ulcer and did not reveal any malignancy.
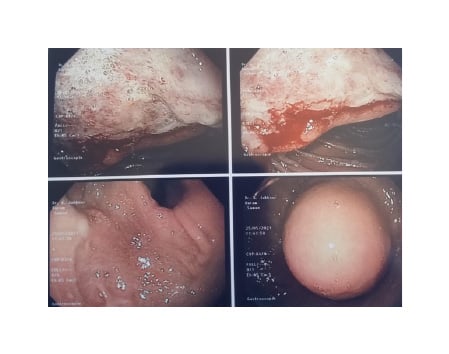
Figure 1: Endoscopic findings of a large 5 cm subepithelial tumour in the gastric pouch above the gastro-jejunal anastomosis with smooth regular borders and a bleeding anastomotic ulcer.
Then, an endoscopic ultrasound was performed (Figure 2A), revealing a 60×59 mm hypoechoic and homogeneous, gastric, subepithelial lesion with regular margins. The lesion originated from the muscularis propria layer of the gastric wall, with a clear cleavage plane separating the tumour from the remnant stomach and adjacent organs. No peri-tumoural lymph nodes were noted. Fine needle biopsy was done, revealing the presence of spindle cell tumour suggestive of GIST, mildly to moderately cellular, without evidence of mitosis, necrosis, or cellular atypia.
This was followed by a CT scan with intravenous and oral contrast (Figure 2B) confirming the presence of a 5 cm heterogeneous mass in the gastric pouch above the level of the gastro-jejunal anastomosis, associated with thickening of the lesser curvature, without contrast enhancement, lymphadenopathy, or distant metastasis. The patient was diagnosed with gastric pouch GIST post-RYGB and weight recidivism.
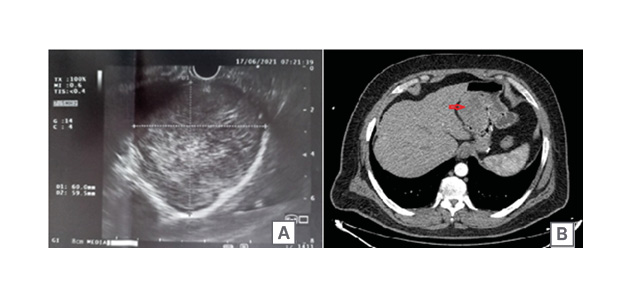
Figure 2: Endoscopic ultrasound (A) and CT scan (B) findings of the tumour.
A) Endoscopic ultrasound finding of a submucosal tumour originating from the muscularis propria layer of the gastric wall, measuring 60.0×59.5 mm, with regular borders. B) CT-scan with intravenous and oral contrast showing the tumour (marked by the red arrow) within the gastric pouch.
THERAPEUTIC INTERVENTION
Consequently, the patient was scheduled for laparoscopic resection of the gastric pouch GIST along with revisional bariatric surgery. Intra-operative findings (Figure 3A) included a large cystic-solid and hypervascular tumour around 6×6 cm in size, occupying most of the gastric pouch, extending proximally, with its epicentre on the lesser curvature of the pouch. The tumour was locally invading the anterior surface of the excluded stomach. Furthermore, a large gastric pouch, an abnormally long blind loop (candy cane), an alimentary limb length of 150 cm, and a biliopancreatic limb length of 100 cm were noted.
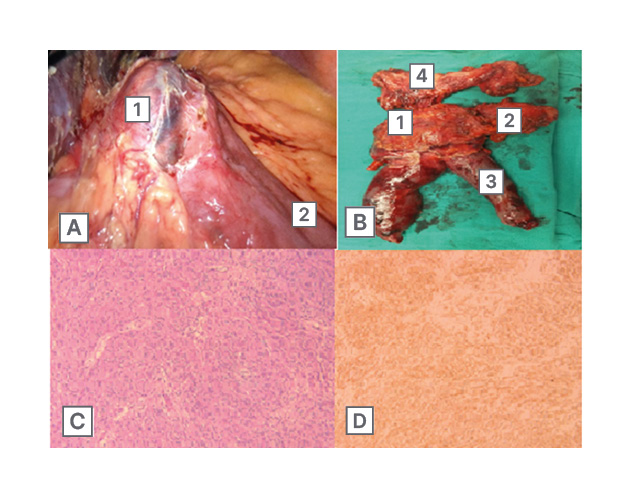
Figure 3: Intra-operative findings.
A) Intra-operative findings of the tumour (1) in the gastric pouch with a relatively long jejunal candy cane (2). B) Post-operative specimen showing the tumour (1) in the gastric pouch (2) with adequate margins, the jejunal candy cane (3), and the remnant stomach (4).
C and D are representative images of the tumour. C) Epithelioid and spindle tumour cells arranged in fascicles stained with haematoxylin and eosin (magnification x10). D) Diffuse membranous and cytoplasmic staining of tumour cells with CD117 (magnification x10).
Laparoscopic en bloc resection of the gastric pouch GIST was performed, with grossly negative margin of 3 cm, incorporating the gastro-jejunal anastomosis and the excluded stomach. Refashioning of a 30 cc gastric pouch was done with a new gastro-jejunal anastomosis (Figure 3B). The post-operative course was uneventful and the patient was discharged on Day 4 after surgery with a gastrografin swallow test negative for leak and stenosis.
Final histopathological examination revealed the presence of a 5.6×5.4 cm mesenchymal neoplasm, which was comprised of epithelioid and spindle cell patterns, presenting three mitotic figures per 50 high power fields, and showing no signs of necrosis. Surgical margins were negative for malignancy. No signs of neoplastic invasions in any of the lymph nodes identified. The tumour was classified as low grade G1 and T3NoMx according to the TNM classification. Immunohistochemistry showed CD17 (+), CD34 (+), and H-caldesmon (+). Molecular studies showed absence of pathogenic variants in PDGFRA exon 12 and 18 in the processed specimen. Because the sample was poor at reception, c-Kit analysis could not be carried out. The above readings established the diagnosis of GIST with epithelioid and spindle cells (Figure 3C and D). A multidisciplinary decision was made to initiate adjuvant imatinib.
FOLLOW-UP AND OUTCOMES
Currently, 1 year post-surgery, the patient is disease-free, with no evidence of local recurrence nor distant metastasis on imaging. They report a weight loss of 28 kg (Table 1).
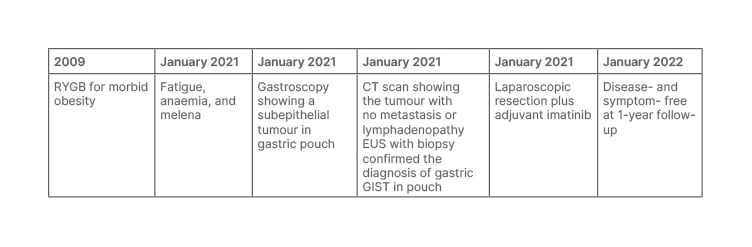
Table 1: Timeline.
EUS: endoscopic ultrasound; GIST: gastrointestinal stromal tumour; RYGB: Roux-en-Y gastric bypass.
DISCUSSION
The first gastric bypass for treatment of morbid obesity was performed in 1966.4 Since then, there has been a dramatic increase in the number of procedures for treatment of obesity. This was followed by the emergence of a minimally invasive approach to perform RYGB, which further escalades the utility of weight loss surgery for treatment of morbid obesity. However, bariatric and metabolic surgery is not a complication-free surgery, and the association between intentional weight reduction through bariatric or metabolic surgery and the reduced cancer risk remains poorly understood. There is limited knowledge and conflicting data in the medical literature, with most studies reporting a decrease in the risk of cancer in patients who undertook weight loss surgery. In fact, merely a few cases of gastric cancer post-bariatric surgery have been reported in the medical literature, with the majority being gastric adenocarcinomas and two cases of gastric GIST, but evidence is lacking concerning the link between gastric cancer and weight loss surgery.1,3
GIST is a rare pathology occurring post-weight loss surgery. Even rarer is gastric pouch GIST. In fact, to the best of the authors’ knowledge, this is the first reported case of gastric pouch GIST post-RYGB. The authors believe that gastric pouch GIST and gastric GIST, in general, after bariatric surgery are a challenge to the unaware. This challenge is partly due to the rarity of this disease and also due to the wide spectrum of presenting symptoms. Having said this, there is an overlap in symptoms between gastric GIST and what can be attributed to normal complaints post-bariatric surgery. In fact, gastrointestinal bleeding is the most common complaint. In addition, patients may report abdominal pain, anaemia, and melena, as seen in this case. Moreover, patients with gastric GIST may be diagnosed incidentally in asymptomatic patients or those complaining of dysphagia, as described by de Roover et al.5-8
The cornerstone for treatment of gastric GIST is surgery with the aim to resect the entire tumour with intact pseudo-capsule, and with special attention not to rupture the tumour as this is directly related to poor prognosis.9 Laparoscopic approach is as effective as the traditional open surgery, especially for tumours that are smaller than 8 cm, and this was the approach in this case.10,11 From here, the prognosis of patients with gastric GIST is satisfactory, and the 5-year survival rate reaches 65%. Factors influencing the prognosis include age, site of tumour, mitotic index, size of tumour, and curative surgery. Furthermore, some studies show the relation between prognosis and immunohistochemistry, such as c-Kit and CD34 positivity, as a possible better prognostic factor.12,13
CONCLUSION
Gastric GIST post-RYGB is a rarely reported entity, and this makes its diagnosis a challenge to the unaware. Furthermore, its rarity dictates absence of universal guidelines. From here, combining the observations of clinicians treating and following up patients post-bariatric surgery in an international database will be beneficial to patients and aid in development of surveillance guidelines.
PATIENT PERSPECTIVE
The patient reports being asymptomatic with a good quality of life.







