Abstract
Background: There is limited literature and a lack of practical guidelines regarding venous thromboembolism (VTE) in patients with acute pancreatitis (AP). The aim of this report is to estimate the prevalence and risk factors of deep vein thrombosis and pulmonary embolism in hospitalised patients with pancreatitis and to evaluate its impact on clinical outcomes.
Methods: A retrospective chart review of patients admitted with AP between 2005 and 2015 was performed. Patients with a secondary diagnosis of VTE were identified. Prevalence and risk factors for VTE development were recorded. The in-hospital mortality rate and length of stay of patients with AP and coexistent VTE was compared with their counterparts without thrombosis. Descriptive statistics and univariate and multivariate analyses were applied where appropriate; p<0.05 was considered statistically significant.
Results: The medical records of 50,564 patients with AP were analysed, with 258 patients (0.5%) presenting concurrent VTE. Factors associated with the development of VTE were length of stay, peripheral arterial disease, malnutrition, and Atlanta systemic complications. Patients with AP and coexistent venous thrombosis showed a significantly higher risk of death (odds ratio: 2.4; 95% confidence interval: 1.51–4.10) and length of stay (22.4 days versus 10.0 days; p<0.001) compared with subjects without thrombosis.
Conclusions: Patients with AP and concurrent thrombosis stay longer in the hospital and have more than a two-fold increase in mortality when compared to the non-thrombotic group.
INTRODUCTION
Acute pancreatitis (AP) is an inflammatory condition of the pancreas that represents one of the most common gastrointestinal cause for hospital admission in high-income countries.1,2 Many European and North American studies have reported a median hospital cost of nearly 7,000 USD per hospitalisation3,4 and 2.6 billion USD per year.5,6 The annual incidence has gradually increased during the past decade. Recent data show that AP incidence varies between 4.9 cases and 73.4 cases per 100,000 worldwide.7,8 Gallstone disease (45%) and a history of excessive alcohol consumption (20%) are the two leading causes of AP.9-11 Pancreatitis clinical outcome is often unpredictable. According to the revised Atlanta classification,12 the severity of AP can be defined as mild, moderately severe, or severe. Most patients run a benign self-limited course and can be discharged within 1 week of admission. However, up to 20% develop local (peripancreatic fluid collections, pseudocysts, pancreatic, or peripancreatic necrosis) and/or systemic inflammatory disease (respiratory, cardiovascular, or renal failure), resulting in complications that pose potential serious problems.13 The overall mortality in patients with AP is 3.5%, while patients with severe AP have a substantial mortality rate of 14–40%.1,2,14,15 Some authors claim that the incidence of early death (within 14 days after admission) does not significantly differ from that of late death (>14 days), organ failure being the cause of death in the early phase (regardless of the presence of necrosis) and infection of pancreatic or peripancreatic necrosis being responsible for mortality in the late phase.16
Vascular disturbances account for 25% of systemic complications in patients with AP, including haemorrhage following an arterial erosion, pseudoaneurysms, and venous thrombosis.17 Although splanchnic vein thrombosis is frequently related to pancreatitis,18-20 deep vein thrombosis (DVT), with or without concurrent pulmonary embolism (PE), in the setting of AP is a rare complication, where incidence remains unknown. As a preventable condition, venous thrombosis prompts a growing interest in AP. The aim of this study is to estimate the prevalence and risk factors of venous thromboembolism (VTE) in AP and to evaluate its impact on clinical outcomes in hospitalised patients with pancreatitis.
MATERIALS AND METHODS
A retrospective chart review of consecutive patients with AP as the primary reason for discharge was performed. The authors identified every patient discharged from an internal medicine department from hospitals in the Spanish Public Health Service (SPHS) between 1st January 2005 and 31st December 2015.
Hospital discharge data were obtained from the Basic Minimum Data Set (BMDS), which is a compulsory registry for each patient admitted to a hospital in the SPHS, a system that cares for more than 90% of the country’s population. As these data are neither identifiable nor private, no institutional review board approval was required. All centres are requested to submit this information to the Spanish Health Ministry. BMDS contains socio-demographic and clinical data for every hospital discharge including gender, age, and, primary and secondary diagnoses, according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code, primary and secondary procedures, admission and discharge status, inpatient stay from the time of admission to discharge, and hospital characteristics (<200 beds, 200–500 beds, 500–1,000 beds, and >1,000 beds).
Patients were selected if they were discharged with the principal diagnosis of AP (ICD-9-CM: 577.00). Patients who had a secondary diagnosis of thromboembolic disease (PE ICD-9-CM: 415.10, 415.11, 415.19; deep venous thrombosis ICD-9-CM: 451.20, 451.81, 451.90, 453.40, 453.41, 453.42, 453.80, 453.90) were analysed.
The following exclusion criteria were used: patients with a previous diagnosis of cancer (ICD-9-CM: 140.00–172.90, 174.00–195.80, 200.00–208.90, V10.00–V10.90), inflammatory bowel disease (ICD-9-CM: 555.00–556.xx), cirrhosis (ICD-9-CM: 572.20–578.00, 456.00–456.29), and a median length of stay less than 2 days.
Definitions
Aetiological factors for acute pancreatitis
Cholelithiasis or choledocholithiasis (gallstone related): ICD-9-CM 574.x0 and 574.x1. Alcohol related: ICD-9-CM 291.xx, 303.xx; 305.0x; 760.71. 980.00, 357.50, 425.50, 535.30, 535.31, 571.00–571.30.
Complications
The grading of the severity in AP has undergone significant recent changes.12,21 In the present study, disease severity was stratified as described in the Atlanta classification22 because it reflects the criteria used in the medical reports and discharge files during the period of the study. Severe AP was defined by the presence of local complications (fluid collections or pancreatic necrosis) and/or organ failure including shock, renal or respiratory failure, or digestive haemorrhage. In the authors’ study, other conditions linked to a poor outcome were also considered as complications during admission.
In order to describe the complications as mentioned above, the authors identified the following ICD-9-MC codes that presented in any secondary diagnosis field in the discharge medical reports: acute respiratory failure (ICD-9-CM: 518.82–518.84), acute renal failure (ICD-9-CM: 403.11, 403.91, 404.12, 585.00–586.00), pneumonia (ICD-9-CM: 480.00–486.00; 003.22, 507.00, 510.00, 510.90, 513.00), bronchoaspiration (ICD-9-CM: 507.00), hypoglycaemia (ICD-9-CM: 251.00–252.00, 250.30–251.00, 250.80–251.00, 249.80–249.81), decubitus ulcer (ICD-9-CM: 707.xx), urinary tract infection (ICD-9-CM: 599.00, 590.xx, 646.60–49, 601.00), sepsis (ICD-9-CM: 531.00–536.00, 537.83, 530.20, 530.82, 038.xx, 995.91, 995.92), gastrointestinal bleeding (ICD-9-MC: 530.21, 530.82, 531.00–535.00, 531.00–535.01, 531.00–535.20, 531.00–535.21, 531.00–535.40, 531.00–535.41, 531.00-535.60, 531.00-535.61), shock (ICD-9-CM: 785.50–785.59), and malnutrition (ICD-9-CM: 260.00–263.90). The presence or absence of complications has been shown in three different ways. Firstly, a composite item including ‘Complications’, if any complication is present. Secondly, a composite variable named ‘Atlanta’, which includes two or more complications linked to severe AP according to the 1992 Atlanta consensus (namely, acute kidney failure, acute respiratory failure, gastrointestinal bleeding, and shock), and finally, every complication in a separate display.
Comorbidity
The Charlson Comorbidity Index (CCI)23 was computed for each patient. This index illustrates the number and relevance of comorbid diseases. It has been used in the present study to adequately depict the presence of additional co-occurring disorders, and thus appropriately adjust the results for the presence of diseases coexisting with AP and VTE that may affect mortality. CCI predicts the 10-year mortality for a patient who may have a range of comorbid conditions. Each condition is assigned a score of 1, 2, 3, or 6, depending on the risk of dying associated with each one. Results provide a total score of 0–37 to predict mortality. A grade higher than 2 is related to a mortality rate >50% per year. Clinical conditions and associated scores are as follows:
- 1 each: myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, chronic lung disease, connective tissue disease, ulcer, chronic liver disease, or diabetes.
- 2 each: hemiplegia, moderate or severe kidney disease, diabetes with end organ damage, tumour, leukaemia, or lymphoma.
- 3 each: moderate or severe liver disease.
- 6 each: malignant tumour, metastasis, or AIDS.
- Length of hospital stay: mean hospital stay was defined as the number of days that each patient spent at the medical centre.
- In-hospital mortality: patients who died during admission were recorded. Deaths that might have occurred after a patient’s discharge were not measured as these data were not available for the investigators.
Statistical Methods
A descriptive analysis was carried out in patients with AP. The demographic variables among patients with or without thromboembolic disease were compared. The authors used the chi-square test for categorical variables with the Yates correction, the Fisher’s exact test for dichotomous variables when the expected value of a cell was less than 5, and Student’s t-test or analysis of variance (ANOVA) for quantitative variables. All the univariate analyses were accomplished after having adjusted for age and gender. The odds ratios (OR) and 95% confidence intervals (CI) were estimated from the regression coefficients.
Univariate analysis was performed to identify variables associated with VTE in patients with PA and with mortality. A multivariate logistic regression analysis was performed to determine the independent effect of diagnosis of VTE on in-hospital mortality. Stratified analyses were performed to examine confounders and interactions. All statistical analyses were carried out with the Statistical Package for the Social Sciences (SPSS, version 16; IBM, Armonk, New York, USA).
RESULTS
There was a total of 50,564 discharges with a primary diagnosis of AP from 2005 to 2015. The average age was 63.4 years (standard deviation [SD]: 18.7 years; range: 17–104 years). Men accounted for 57.3% of the patients. The median hospital stay was 98 days (SD: 10.5 days; range: 2–357 days). A CCI >2 was present in 5.4% of the cases. The average cost was 4,519.8 EUR (SD: 5,049.9 EUR; range: 1,944.4–119,417.0 EUR). During admission, 7.3% of patients developed a severe AP as described in the Atlanta classification. All-cause mortality in patients with AP was 2.9%. A total of 258 patients (0.5%) were diagnosed as having concurrent VTE. Among patients with VTE, isolated DVT was found in 198 (76.7%), while PE alone was diagnosed in 54 (21%). Both DVT and PE presented simultaneously in 6 (2.3%) patients.
Within the study period, an increasing temporal tendency was seen in AP prevalence, from 3,926 (7.8%) cases in 2005 to 4,929 (9.7%) cases in 2015, although the statistical analysis failed to show a trend significance. VTE prevalence showed an irregular pattern throughout the period of study, varying from 0.2% to 1.0%, depending on the year. Similarly, variation in mortality prevalence ranged from 2.5% to 3.3% throughout the study interval, both without a significant trend.
In the univariate analysis, patients with AP and concurrent VTE had a significantly higher length of stay (22.4 days versus 10.0 days; p<0.001), There was also a higher percentage of peripheral arterial disease (9.3% versus 3.4%; p<0.001), and chronic obstructive pulmonary disease (21.7% versus 12.8%; p=0.002). Furthermore, the following complications were more frequently reported in patients who developed VTE: sepsis (5.0% versus 1.2%; p<0.001), pneumonia (3.1% versus 1.1%; p=0.014), malnutrition (7.7% versus 1.3%; p<0.001), acute renal failure (10.0% versus 5.2%; p=0.002), acute respiratory failure (10.8% versus 2.5%; p<0.001), systemic inflammatory response syndrome (1.5% versus 0.2%; p<0.005), and the presence of more than one systemic complication as defined in the Atlanta criteria (23.2% versus 11.0%; p<0.0001). In the multivariate logistic regression analysis, the demographic and clinical factors that were independent predictors of occurrence of VTE in patients with AP were length of stay, peripheral arterial disease, malnutrition, and the combination of two or more Atlanta systemic complications (Table 1).
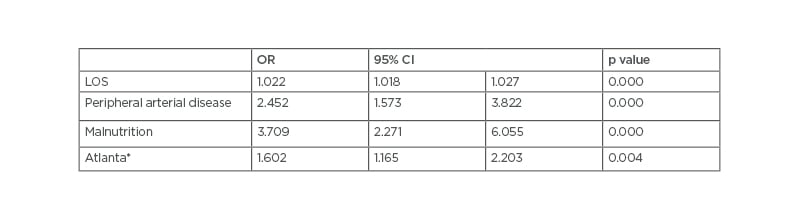
Table 1: Multivariate analysis of factors associated with venous thromboembolism in acute pancreatitis.
*At least two systemic complications
CI: confidence interval; LOS: length of stay; OR: odds ratio.
Table 2 shows the covariates that were significantly associated with mortality in the univariate analysis. Patients who died were more frequently women (59.2% versus 42.1%; p<0.001) and older (82.8 years versus 62.4 years; p<0.001). Higher in-hospital mortality was also observed in patients with more comorbid conditions (CCI>2: 14.5% versus 5.1%; p<0.001), in participants with VTE (1.6% versus 0.04%; p=0.001), and in those who presented more AP-related complications such as shock (7.4% versus 0.4%; p<0.001), acute kidney failure (31.6% versus 4.5%, p=0.02), and acute respiratory failure (24.1% versus 1.9%; p=0.001).
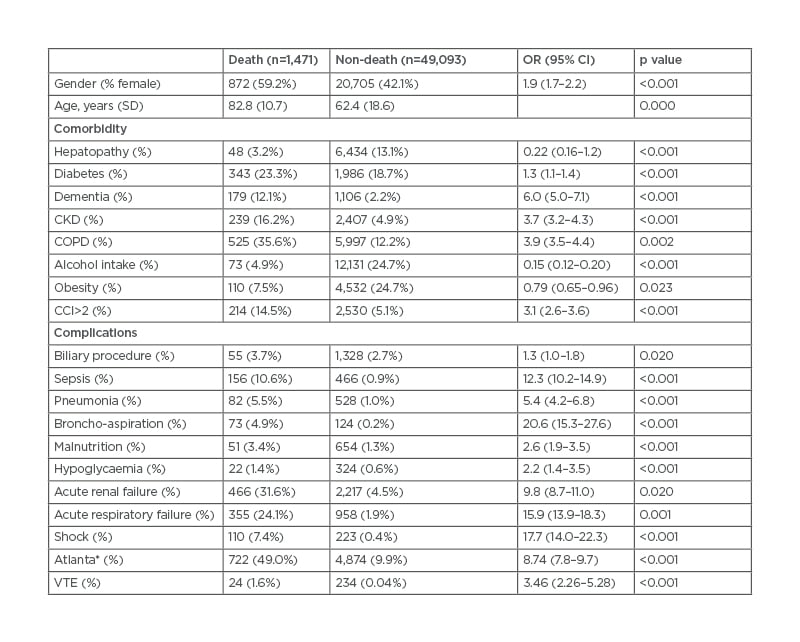
Table 2: Univariate analysis of factors associated with mortality in acute pancreatitis.
*At least two systemic complications.
CCI: Charlson Comorbidity Index; CI: confidence interval; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; OR: odds ratio; SD: standard deviation; VTE: venous thromboembolism.
A multivariate logistic regression analysis was performed to further assess which variables were independently associated with mortality (Table 3). The factors that remained as independent predictors of mortality in patients with AP were female gender (OR: 1.21; 95% CI: 1.08–1.36), age (10 years) (OR: 4.47; 95% CI: 4.06–4.93), CCI (OR: 1.50; 95% CI: 1.27–1.78), two or more Atlanta complications (OR: 5.06; 95% CI: 1.27–1.78), pneumonia (OR: 2.36; 95% CI: 1.78–3.11), systemic inflammatory response syndrome (OR: 6.14; 95% CI: 3.97–9.49), sepsis (OR: 4.76; 95% CI: 3.82–5.93), and VTE (OR: 2.48; 95% CI: 1.50–4.10). Overall, mortality in patients with AP was 2.9%; however, when analysed separately according to the presence of VTE, the results revealed that 7.5% of patients with AP and coexistent VTE compared with 2.9% of their counterparts without VTE died.
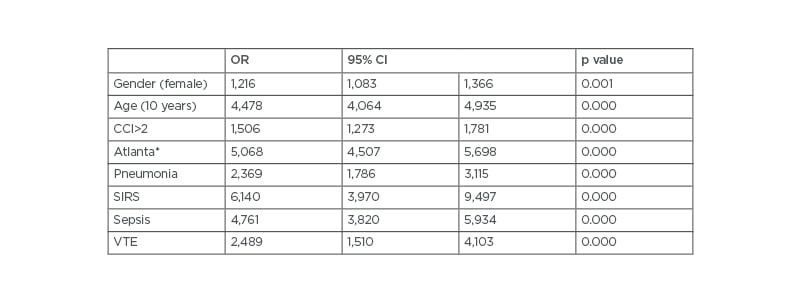
Table 3: Multivariate analysis evaluating variables independently associated with in-hospital mortality in all patients with acute pancreatitis.
*At least two systemic complications
CCI: Charlson Comorbidity Index; CI: confidence interval; OR: odds ratio; SIRS: systemic inflammatory response syndrome; VTE: venous thromboembolism.
DISCUSSION
Despite improvements in diagnostic techniques, antibiotic therapy, surgical treatment, and critical care, AP is an unpredictable condition that continues to be associated with high mortality rates in severe cases, mainly related to organ failure and infection of pancreatic or peripancreatic necrosis.14,15 Among vascular systemic complications, venous thrombosis is also associated with adverse outcomes in hospitalised patients with AP.17,24 Following close anatomical ties with the pancreas, the most common venous vascular complication in pancreatitis involves the splanchnic veins including portal vein, splenic vein, and superior mesenteric vein, either separately or in combination.19,20
Portosplenomesenteric vein thrombosis may lead to portal hypertension, with high risk of gastrointestinal bleeding, bowel ischaemia, intra-abdominal haemorrhage, ascites, splenomegaly, and splenic infarction, among other complications25-27 While splanchnic vein thrombosis is a well-known phenomenon in pancreatitis, VTE is a rare complication and is less commonly reported. To date, very little data is available on the prevalence of pulmonary and deep veins thrombosis in hospitalised patients with pancreatitis. Prior publications of VTE in the setting of AP are mostly case series reports28-33 and two population-based analyses including information of inpatient databases similar to the authors’ cohort study.35,35
In general population, both PE and DVT have an overall incidence of 0.1% per year, while PE and DVT inpatient incidence increases up to 0.4% and 1.3%, respectively.1 In the present report, the prevalence of VTE among patients with AP was 0.5%. Previous analyses have stated different results depending on the patient selection. Studies including only patients suffering with necrotising pancreatitis have noted a significantly higher prevalence of DVT (16%)37 while reports with all degrees of pancreatitis severity show a similar VTE prevalence as the one described in the authors’ study and are in concordance with the prevalence in other hospitalised patients.34
The potential specific mechanisms that may account for the development of VTE in patients with pancreatitis remain unclear. Immobilisation in prolonged hospitalised patients, regardless of the reason for admission, is a recognised mechanism for venous stasis.38 This condition has also been reported in patients with pancreatitis for, even in its mildest clinical presentation, patients admitted with AP stay up to 5 days in the hospital.39,40 Several specific explanations have been proposed to elucidate the development of VTE in patients with AP. The systemic inflammatory response associated with pancreatitis induces endothelial damage at a microvascular level, resulting in a pro-thrombotic state and making vascular events more likely.33 Besides, the release of pancreatic proteolytic enzymes into the vessels may provoke a procoagulant state leading to venous thrombosis.41 Furthermore, the mass effects from the surrounding inflamed pancreas may also contribute to a prothrombotic milieu, especially in splanchnic veins.26
In this analysis, several parameters have been identified as independent predictors for VTE among hospitalised patients with AP, namely median hospital stay, peripheral arterial disease, malnutrition, and systemic organ dysfunction. These findings agree with previous studies and are consistent with the fact that sicker patient profiles develop more frequently into thrombotic events.42 Similarly, it has been reported that patients with more complicated pancreatitis are more prone to present simultaneous VTE.34 It is not surprising that in the authors’ study, patients with serious comorbid conditions such as peripheral arterial disease and those who run a complicated pancreatitis course with organ failure present more frequently concurrent venous thrombosis.
The authors’ results show that VTE is adversely associated with mortality. Patients with pancreatitis and coexistent VTE die approximately twice as much as their counterparts without VTE (7.5% versus 2.9%). Likewise, the current study reveals that length of stay is significantly higher in patients with AP and concurrent VTE (22.4 days versus 10.0 days). Prior retrospective studies also point out that VTE in patients with AP is associated with adverse outcomes.34
The major strength of the authors study is the fact that it includes a large nationwide population (more than 50,000 patients), allowing statistically precise estimates of the prevalence and relationship of VTE with adverse disease course in patients with pancreatitis. Administrative databases provide massive information not only for reimbursement purposes but also for clinical research43-45 and, despite some methodological limitations, databases are increasingly used in public health research.46 Compared with previous reports, this is a population-based analysis involving a large cohort of patient records and thus adequately powered to detect differences between thrombosis and non-thrombosis groups.
There are, however, certain caveats that may affect the results. First, the main limitation is its retrospective nature. Data have been fully obtained from the BMDS administrative database and, therefore, the authors’ findings are subject to information bias. Erroneous clinical documentations can lead to misclassification. However, this system has long been accepted in many different countries in the authors’ environment. Many authors have examined in previous reports data from large national and multinational databases including information on patients’ discharge records.34,47-49 A second limitation is that the relationship between the occurrence of a venous thrombotic event and the presence of pancreatic necrosis has not been evaluated. Little data is available regarding DVT and PE and necrotising pancreatitis; however, some studies on the association between venous thrombosis and pancreatitis show that splanchnic vein thrombosis is significantly higher in patients with pancreatic necrosis.50-52 Administrative data use codes to identify diagnosis or procedures. Based on ICD-9-CM 577.0, a ‘grouper’ programme assigns a DRG 204 to all patients with AP, irrespective of the presence of necrosis. Therefore, this administrative classification does not allow adjustment for oedematous or necrotising pancreatitis. The third limitation is that no information is available regarding patients who developed VTE despite the use of prophylactic anticoagulation or those who were under pro-thrombotic treatments.
Finally, another weak point is that the diagnostic means of VTE have not been recorded (doppler ultrasonography or contrast-enhanced CT scanning), which can significantly change the incidence of VTE in patients with AP.53
The following conclusions can be drawn from the present report. Firstly, it is known that VTE is a frequent condition in hospitalised patients. Nearly 25% of all thromboembolic events occur during or are related to a recent hospitalisation.54,55 Thromboembolic complications are associated with high mortality and morbidity and with an increased consumption of healthcare resources, leading to significant associated costs.65 These findings in medical hospitalised patients have been confirmed in the authors’ report: the development of VTE increases both mortality and median hospital stay in patients with pancreatitis. Secondly, VTE is a potentially avoidable complication and is responsible for approximately 10% of deaths within the hospital. It has become the leading cause of preventable death in hospitalised patients. The present study may help to recognise patients who might benefit from mechanical or pharmacological thromboembolic prophylaxis. Several VTE risk factors in hospitalised patients with pancreatitis have been identified, such as length of stay, peripheral arterial disease, malnutrition, and systemic organ dysfunction. The authors’ data show that sicker patients with pancreatitis present higher prevalence of thrombotic events; thus, management strategies to decrease and control organ dysfunction in pancreatitis may reduce the development of VTE and, therefore, may help improving healthcare resource utilisation.
Prospective studies have shown that DVT and PE incidence in hospitalised patients who do not receive thromboprophylaxis can reach 15.0% and 1.5%, respectively.58-60 Pharmacological prophylaxis with heparins is safe and effective, with reductions in DVT and PE relative risk of 40–70%.61,62 The American College of Chest Physicians (CHEST) guide for the prevention of VTE in non-surgical patients recommends the use of low-molecular-weight heparin, unfractionated heparin, or fondaparinux, unless contraindicated.63 However, thromboprophylaxis may also increase haemorrhagic complications in acutely ill patients with pancreatitis undergoing invasive procedures. Lacking a standard of care in the current clinical practice, the decision to use pharmacological prophylaxis is made on a case-by-case basis. Further research is needed to determine the specific recommendations for VTE prophylaxis in patients with pancreatitis deemed high risk.








