Abstract
Since it was first identified in 1982, Helicobacter pylori has continued to draw attention far beyond its role in peptic ulcer disease and is now associated with a myriad of immune-mediated diseases, both inside the gastrointestinal tract (GIT), such as mucosa-associated lymphoid tissue lymphoma, and systemic diseases, such as H. pylori-associated immune thrombocytopenia. This association has ignited research into the mechanisms of H. pylori pathogenicity, especially regarding its role within a multitude of diseases outside the GIT. Despite controversies, a growing body of evidence has begun to establish potential associations between H. pylori and extragastric GIT pathologies; H. pylori has recently been associated with luminal diseases, such as inflammatory bowel diseases and coeliac disease, as well as pancreatic, hepatobiliary, and malignant diseases of the GIT. Despite the lack of conclusive evidence regarding the mechanisms of these relationships, studies have found strong associations, like the case of H. pylori and coeliac disease, while others have not discovered such connections. In addition, while studies have established positive associations between H. pylori and various extragastric diseases, other studies have found the pathogen to play a protective role in disease development. This review comments on the latest evidence that addresses the role of H. pylori in non-gastric gastrointestinal diseases, and establishes the nature of these relationships and the implications of H. pylori eradication from a clinical perspective.
INTRODUCTION
Helicobacter pylori is a curved, Gram-negative bacillus that colonises the gastric mucosa, usually during childhood, and can persist throughout the host’s life without requiring intervention.1,2 The prevalence of H. pylori in the adult population of north Europe and the USA is approximately 30%, compared to a higher range of 40–80% reported in adults living in or immigrating from south and east Europe, South America, and Asia.3 Moving away from its most well- known role in gastritis and peptic ulcer diseases,4,5 increasing evidence focusses on H. pylori-associated extragastric manifestations within the gastrointestinal tract (GIT).
HELICOBACTER PYLORI AND INFLAMMATORY BOWEL DISEASES
Cases of inflammatory bowel disease (IBD), consisting mostly of ulcerative colitis and Crohn’s disease (CD), are immune-mediated chronic inflammatory diseases of the bowel. The exact pathogenesis of IBD is unknown; however, it is theorised to result from a dysregulated immune response to host intestinal microflora among genetically susceptible individuals.6
Recent data focussing on H. pylori involvement in the pathogenesis of IBD is gaining attention.6-9 Various mechanisms have been proposed that link H. pylori to IBD, including induction of alterations in gastric and intestinal permeability via various immunological pathways, or via induction of antigenic material absorption with subsequent loss of self-tolerance.10 In the context of gastritis, the histopathology of the gastric mucosa infected with H. pylori is understood to strongly stimulate mucosal inflammation, thereby causing an inflammatory response characterised by an influx of neutrophils, mononuclear cells, and T helper 1 (Th1) cells that destroy intracellular pathogens.4,11,12 Furthermore, H. pylori interaction with the gastric epithelium induces a proinflammatory response, including the recruitment and upregulation of chemokines and cytokines. However, as H. pylori does not exist intracellularly, the Th1 response results in endothelial damage to the host’s gastric mucosa and is incapable of pathogen clearance.13
Several studies have reported that the prevalence of H. pylori infection is lower in patients with IBD compared to controls,7,8,10,14,15 and this inverse relationship prompted investigations and shifted the focus of research to the potential protective role of H. pylori in the development of IBD. By colonising the gastric mucosa, H. pylori has acquired a series of attributes, primarily a downregulation of the host’s immune system to evade both the innate and acquired immune system and prevent pathogen clearance.4,12 While the mechanisms of proinflammatory states are linked to the development of gastritis and other inflammatory diseases, the long-term, sustained relationship between H. pylori and the human host raises the possibility of an existing symbiotic relationship, in which its persistence in the GIT may be beneficial to some individuals. Through these proposed mechanisms, it has been suggested that H. pylori plays a protective role in some immune-mediated and allergic diseases.11,12,16-18
A meta-analysis of the prevalence of H. pylori among IBD patients compared to their control subjects showed a lower prevalence of H. pylori infection among IBD patients.10 Another meta-analysis, including 4,400 IBD patients and 4,763 controls, resulted in a prevalence of 26.0% versus 44.7% of H. pylori positivity in the IBD versus control group, respectively.19 This inverse relationship may have some alternative explanations; firstly, it has been suggested that treatment with sulfasalazine, or the long-term and wide-spectrum antibiotic therapy among IBD patients, could be responsible for the eradication of H. pylori.6,20 Notwithstanding this, a recent meta-analysis found that the protective effect of H. pylori was not influenced by previous use of amino salicylates or corticosteroids; however, antibiotics amplified this negative association.7 Secondly, it is possible that immunological alterations of the gut mucosa induced by IBD could prevent H. pylori colonisation.8 Lastly, it has been proposed that H. pylori infection shifts the equilibrium between Th1 and Th2 immune responses to a Th2-dominant pattern.
In addition, since the eradication of H. pylori increases the levels of Th1 proinflammatory cytokines, genetically susceptible individuals are predisposed to CD.14 With respect to Th1 and Th2 immune responses, data are limited on the prevalence of virulent H. pylori strains among IBD patients; however, one study showed that both CD patients and controls were equally infected by H. pylori seropositive for cytotoxin-associated antigen A (Cag A), demonstrating no correlation between virulent H. pylori strains and the risk of IBD.6 Furthermore, Engler et al.20 investigated the possible protective effects of H. pylori by administering regular doses of H. pylori extract into dextran sodium-sulfate-induced chronic colitis mouse models. The results demonstrated that this experimental infection with H. pylori in mouse models alleviated clinical and histopathological features of chronic colitis and T cell transfer-induced colitis, and proposed that H. pylori can be exploited for the prevention and/or treatment of IBD through its immunomodulatory activity.18,20 Taken together, this evidence indicates that there is a relatively strong negative association between the presence of H. pylori and IBD, whereas direct inhibition of IBD by H. pylori remains to be determined in humans.
HELICOBACTER PYLORI AND MICROSCOPIC COLITIS
Microscopic colitis is an umbrella term consisting of chronic colonic inflammation characterised by a normal macroscopic appearance of the colonic mucosa, contrasted by the presence of intraepithelial lymphocytic infiltrate or subepithelial collagen bands on biopsy. The primary cause of microscopic colitis is not fully understood, but it has been suggested to be a result of an immunological reaction to luminal antigens of ileal origin.21,22
Current data support an inverse relationship between H. pylori and microscopic colitis, similar to that seen in IBD. While Sonnenberg et al.23,24 also established a negative correlation between microscopic colitis and H. pylori, another potential factor was exposed. The prevalence of H. pylori was shown to vary among ethnic groups, which in turn directly affected the prevalence of microscopic colitis among patients. For example, where H. pylori was prevalent within the Hispanic populations, there was a notable reduction in the incidence of microscopic colitis, whereas there was an increased prevalence among Caucasians and African-American populations. This inverse relationship established between H. pylori and microscopic colitis may shed light on the ethnic variations in H. pylori gastric colonisation and may be partly responsible for the observed ethnic distribution of microscopic colitis.23,25 Research addressing the associations between H. pylori and microscopic colitis remains limited; to gain further insight into the precise role of H. pylori in microscopic colitis pathophysiology, additional research is warranted.
HELICOBACTER PYLORI AND COELIAC DISEASE
Coeliac disease, also known as gluten-sensitive enteropathy, is an autoimmune disease involving interactions between environmental, genetic, and immunologic factors; specifically, it is defined as a T cell-mediated immune disorder that is induced by gluten ingestion in genetically predisposed individuals.26,27 There has been a significant increase in the incidence of coeliac disease in recent decades,28 rather than those cases recorded solely as a by-product of improved and increased detection. This trend is not well understood; however, environmental factors, specifically a reduced exposure to bacteria, also known as the ‘hygiene hypothesis’, have been proposed to be reponsible.29 As part of an analysis of a nationwide pathology database, Lebwhol et al.29 found a strong inverse relationship between the presence of H. pylori and coeliac disease, independent of confounding factors. Although the potential protective mechanism is uncertain, this study is consistent with other studies attributing the role of H. pylori with a decreased risk of allergic and inflammatory conditions.18
In 2015, Robinson et al.5 conducted a systematic review on the protective role of H. pylori against the risk of acquired autoimmune disorders. Where some studies showed that the prevalence of H. pylori was reduced in coeliac disease, other reports found no differences. While these controversies could be explained by research methodologies, biases, or confounders, the mechanisms by which H. pylori might protect against CD remain unknown. Shortly after this review, Rostami-Nejad et al.30 reviewed a series of reports addressing pathological and clinical correlations between coeliac disease and H. pylori infection. A comprehensive review of research articles from 1985–2015 concluded that examinations of the correlation between H. pylori and CD have once again yielded discordant outcomes.30
More recently, Basyigit et al.31 failed to confirm a significant relationship between coeliac disease and H. pylori. Serum levels of coeliac disease-specific autoantibodies or immunoglobulin A were analysed among both H. pylori and non-H. pylori-infected individuals and no significant differences in serum level antibodies were found. Conversely, Narang et al.32 concluded that the prevalence of H. pylori in children with confirmed coeliac disease was lower in comparison to H. pylori infection rates in children without coeliac disease, signifying an inverse relationship. While protectivity has still not been confirmed, the potentiality now merits further longitudinal investigations to ascertain whether H. pylori infection confers protection or a reduced risk of developing coeliac disease.
HELICOBACTER PYLORI AND HEPATOBILIARY DISEASES
Early research suggests the Helicobacter species is implicated in hepatobiliary diseases, ranging from chronic cholecystitis and primary sclerosing cholangitis (PSC), to gallbladder and primary hepatic carcinomas (Table 1).17,69,70 The Helicobacter species, including H. pylori, has been implicated as a potential inducer of hepatocyte and biliary autoimmunity, primarily based on their tolerance to, and ability to survive in, bile.71
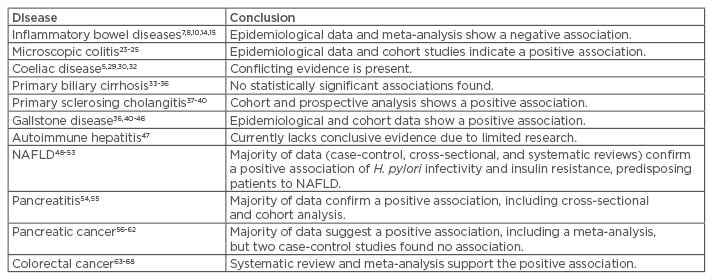
Table 1: Extragastric gastrointestinal associations of Helicobacter pylori.
NAFLD: non-alcoholic fatty liver disease.
However, H. pylori primarily affects the GIT and is not commonly present in the liver and bile ducts of H. pylori-infected individuals. Contamination of the hepatobiliary system is most likely due to contamination from potentially damaged gastrointestinal epithelium, or to the dysfunctional sphincter of the common bile duct; however, the entry of H. pylori through this mechanism, and the susceptibility of biliary tract contamination, is not limited to H. pylori. This presents challenges in the assessment and controlled research concerning the role of H. pylori in the development of hepatobiliary pathologies.
There is minimal evidence of association pertaining to the role of H. pylori in autoimmune biliary diseases, including primary biliary cirrhosis (PBC) and PSC.33,71
As both conditions are caused by an immune-mediated cellular destruction within the biliary system, research into the implication of H. pylori infectivity within the pathophysiology of these diseases has recently gained attention.
Primary Biliary Cirrhosis
While certain attempts have failed to detect H. pylori in the livers of patients with PBC,33 others have detected H. pylori in a very limited number of samples.34 Studies, such as that by Boomkens et al.,70 could only find the presence of Helicobacter species in approximately one-third of all samples tested, which suggested that PBC is unlikely to be caused or influenced by Helicobacter infection. Additionally, research specifically assessing Cag A seropositivity of H. pylori in PBC patients compared to control groups also found conflicting evidence.33,34,71 Research on molecular mimicry between H. pylori and PBC-specific autoantigens identified a significant amino acid sequence similarity between the mitochondrial autoepitope region of pyruvate dehydrogenase complex E2 subunit and urease beta of H. pylori, without any evidence of immunological cross-reactivity. Other studies involving cross-reactive antibodies against H. pylori also demonstrated that H. pylori antigens are unlikely candidates of cross-reactive targets in molecular mimicry mechanisms in PBC, contributing further to the evidence against H. pylori association with PBC.35
Primary Sclerosing Cholangitis
In comparison to PBC cases, there was some evidence identifying a positive association between PSC and H. pylori. A study on H. pylori DNA in liver samples from PSC patients detected H. pylori DNA in micro-dissected hilar biliary epithelium in a higher number of PSC patients compared to controls.35 This supports the hypothesis that bile reflux, or translocation from the duodenum into the biliary tract, might transport H. pylori organisms into the biliary system, contributing to PSC development. Gut translocation of pathogens has been suggested among several studies as a plausible mechanism for the induction of hepatobiliary autoimmunity within the context of H. pylori involvement in hepatobiliary pathology.37,39,71 Although several studies assessing the prevalence of anti-H. pylori antibodies in PSC patients found that titres did not differ significantly between PSC patients and control groups, it has been hypothesised that, since many PSC patients suffer from ulcerative colitis, H. pylori could account for any documented associations.36-38 Thus far, a positive association between H. pylori and PSC has been elucidated; however, the attempts made to uncover any potential association between H. pylori and PSC have been limited, thereby requiring further clarification.
Gallstone Diseases
H. pylori is positively associated with cholestatic conditions, albeit with limited evidence.34,38 It has been proposed that H. pylori plays a role in the formation of cholesterol gallstones, and consequentially chronic cholecystitis.39 For example, Attaallah et al.41 found 37% of patients with symptomatic gallstone disease were locally infected with H. pylori. Other studies also found H. pylori to be a predisposing factor and showed that it may have a pertinent role within the aetiology and pathophysiology of gallstone formation;40,42-44 no evidence was found for any negative or non-association in our literature review. Although causality is uncertain at this point, H. pylori plays a role in gallstone pathogenesis, either directly or as a potential cofactor.45
Autoimmune Hepatitis
Although a high prevalence of H. pylori infection was demonstrated in patients with viral-related cirrhosis, it was not studied in cases of autoimmune hepatitis. Durazzo et al.,46 in their attempts to establish a relationship between autoimmune hepatitis and H. pylori, found no association. Current data on the role of H. pylori in autoimmune hepatitis are limited and inconclusive.
Helicobacter Pylori and Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) is regarded as a hepatic manifestation of metabolic syndrome. It is a concerning disease, not only because of its high prevalence, but also due to its potential risk of progressing to cirrhosis and hepatocellular carcinoma. The literature focussing on the association between H. pylori and NAFLD is conflicting; as we have established, H. pylori is a pathogen reported to be well associated with metabolic syndrome,48 but whether there is a direct association with regard to NAFLD still remains unclear.49,50
Okushin et al.,49 in a cross-sectional study, aimed to clarify the nature of association between NAFLD with causative background factors, including H. pylori infection. While BMI, serum alanine aminotransferase, and platelet count were significantly associated with NAFLD, H. pylori infection was not.49 However, others have found H. pylori to be an independent risk factor for the development of NAFLD.50,51 Cheng et al.,50 in a systematic review of H. pylori involvement in the pathogenicity of NAFLD, revealed the possible contributory mechanisms of H. pylori to the disease: H. pylori infection causes chronic low-grade systemic inflammation, which increases the levels of inflammatory cytokines, and, through a series of pathways ultimately leading to insulin resistance, acts as a leading contributor to NAFLD.48,50 Lastly, Tang and Kumar53 stated that small trials examining the effect of H. pylori eradication have shown improvement in markers of NAFLD activity. Not only does this support a link between the two conditions, but it substantiates the potential clinical implications beyond H. pylori eradication in patients with NAFLD.
HELICOBACTER PYLORI AND PANCREATITIS
Pancreatitis can be of acute, chronic, and autoimmune nature, each displaying differences in pathophysiology. Acute pancreatitis is defined as an acute inflammation of the pancreas, consequentially causing a dysregulation of pancreatic enzyme activity, and the pathogenesis ranges from hereditary and behavioural, to environmental aetiologies.16 In contrast, chronic pancreatitis is a more progressive fibro-inflammatory disease, characterised by irreversible fibrosis of the pancreatic gland,47 and autoimmune pancreatitis (AIP) is characterised by fibrotic, lymphoplasmacytic inflammatory processes accompanied by T cell apoptosis, which contribute to pancreatic tissue destruction.17
The implication of H. pylori in the pathogenesis of AIP via an induction of autoimmunity has been a recent topic of investigation. The role of H. pylori in AIP has also been investigated within the context of a high prevalence of peptic ulcer disease (PUD) in patients with AIP.16,72 Lee et al.72 found PUD to be positively associated with severe acute pancreatitis, thereby concluding that there are implications beyond the treatment of PUD, and the condition should be considered in the management of acute pancreatitis. Another study found that H. pylori increases the severity of ischaemia-induced pancreatitis, as well as inducing production of proinflammatory interleukin-1β, further aggravating disturbances in the pancreatic microcirculation in acute pancreatitis.54 Lastly, H. pylori may potentiate the onset of AIP due to molecular mimicry; research investigating this molecular mimicry found H. pylori alpha carbonic anhydrase and human carbonic anhydrase II, among other enzymes, were highly expressed in the pancreatic ductal and acinar cells, exposing the immune system to the potential of cross-reactivity.55 While the exact mechanisms are still unknown, molecular mimicry, proinflammatory response, and the development of H. pylori-induced PUD may be involved in the development of AIP.
There is a positive association between pancreatitis and H. pylori; the evidence against any association between the two was not found to be statistically significant. Whether this relationship is incidental, causative, or a risk factor remains to be understood. The question concerning the implications of eradication of H. pylori to patients with AIP, in terms of the potential benefits, is now becoming more relevant and, therefore, as we consider the increased burden of pancreatic cancer and its association with AIP, the development of an underlying link motivates further research.
HELICOBACTER PYLORI AND PANCREATIC CANCER
Pancreatic cancer is currently the fourth leading cause of cancer-related deaths worldwide and the risk factors include tobacco, smoking, diabetes, chronic pancreatitis, alcohol, and specific genetic conditions.73 Recently, research focussing on the associations between H. pylori and autoimmune diseases has found conflicting evidence for H. pylori involvement in pancreatic cancer. Several studies have established a positive association between H. pylori and pancreatic cancer.56-58 For example, Risch et al.57 found that there is evidence for the involvement of H. pylori-modulated gastric acidity in the risk of pancreatic carcinoma. Additionally, the interaction of the ABO genotype and phenotype status of an individual influences the behaviour of H. pylori infectivity, which in turn affects gastric and pancreatic secretory function. This ultimately influences the pancreatic carcinogenicity of dietary and smoking-related N-nitrosamine exposures.56,59 The association of ABO-product antigen and H. pylori is now understood to be strongly implicated in the disease aetiology of pancreatic cancer. Furthermore, a meta-analysis including 1,003 pancreatic cancer patients and 1,754 healthy controls evaluated the presence of H. pylori and the risk of developing pancreatic cancer. The results indicated a significant correlation between H. pylori and pancreatic cancer, and showed that H. pylori infection can increase the risk of developing pancreatic cancer.59 With regard to Cag A seropositivity, Stolzenberg-Solomon et al.69 found that, compared with seronegative subjects, those with H. pylori or Cag A+ strains were at an elevated risk of developing pancreatic cancer.
However, several other studies found no association between H. pylori and the development of pancreatic cancer.60,61 Both a case-control study by de Martel et al.61 and a meta-analysis by Xiao et al.74 found no associations between the presence of H. pylori or the Cag A protein and pancreatic cancer, whereas smoking and education were associated. Due to the limited number of studies, suspected publication bias, relatively small sample size, as well as statistical heterogeneity, the need for high-quality studies to further clarify the role of H. pylori in pancreatic cancer still stands.
HELICOBACTER PYLORI AND COLON CANCER
Colorectal cancer is the third most common cancer worldwide, accounting for >9% of all cancer incidences.75 Although colonic carcinogenesis is considered a multifactorial process, including a combination of environmental factors, Western dietary and social practices, as well as genetic susceptibility, the precise mechanisms of its initiation remain unknown. Given the increased incidence of colon cancer worldwide, as well as a growing interest in the involvement of H. pylori in gastrointestinal diseases, studies have begun to examine the relationship, or potential association, between H. pylori and colonic carcinogenesis.63
Until now, the role of H. pylori in the development of colorectal neoplasm remains controversial. A study that involved mice infected with H. pylori exhibited oncogenic properties within the colon epithelia, including an increased expression of mutations.64 Selgrad et al.65 found an upregulation of Cag A expression to be associated with an increased risk of colon cancer, also associating infectivity with H. pylori to the development of colorectal cancer. A broader and more recent study in China, based on a retrospective analysis of intestinal biopsies, revealed that interstitial metaplasia accompanied by H. pylori infection was significantly associated with an increased risk of adenomas, suggesting a plausible carcinogenic effect of H. pylori.66 Several large-scale studies, which included data from several case-control studies, cross-sectional studies, and meta-analyses, found a similar statistically significant association between H. pylori and the risk of colorectal adenoma and colorectal neoplasm.66-68 However, it still remains to be elucidated whether the induction and perpetuation of inflammatory responses, alteration of gut microflora, and release of toxins mediated by H. pylori can claim causality to colorectal tumourigenesis.76 While the evidence thus far indicates an association, rather than a proof of causality, it calls for further large-scale studies to confirm H. pylori as a contributor in the pathogenesis of colorectal cancer.
CONCLUSION
H. pylori is a pathogen that has continued to draw attention for decades. Thus far, despite the increasing evidence of both positive and negative associations of H. pylori and extragastric diseases, specifically within gastrointestinal and hepatobiliary systems, the direct role of the pathogen in the pathophysiology of the aforementioned diseases remains elusive. The main protective effect is postulated to result from the long-term interaction of H. pylori with the immune system, acquired in early childhood. Evidence suggesting whether H. pylori eradication truly reverses the immunological benefits is lacking; however, evidence regarding the benefits of eradication exists. The lack of a concise and comprehensive understanding makes the implications of H. pylori infectivity unclear from a clinical standpoint. Lastly, although H. pylori is both a friend and a foe, currently there is no evidence against treating a patient with H. pylori when there is a strong clinical indication for therapy.








