Abstract
It was predicted that the biological era might alter the natural history of Crohn’s disease, preventing the well-documented inflammation–fibrosis–fistulisation sequence. However, despite the development of novel biological therapies, average efficacy at 1 year remains at 30–50%, with this number decreasing as second-line therapies are regularly required. Currently, new advanced therapies are under investigation to provide alternatives to available treatments. In addition, novel, nonpharmacological strategies are also being explored. Surgical intervention, currently inevitable in a significant proportion of patients, necessitates that prevention of postoperative recurrence is an important research focus and recent studies have shed light on the long-term efficacy of early operative interventions and emerging surgical techniques. Cellular therapies, including stem cell therapy for perianal disease, stem cell transplantation, and harnessing the therapeutic potential of regulatory T cells, are in various stages of development. These could conceivably change the landscape of Crohn’s disease management. Dietetic interventions offer a lower risk alternative that may be used as an adjunct to other therapies or where immunosuppression is unfavourable. Choosing between these therapeutic options depends on multiple factors, including the associated risk–benefit profile, available alternatives, as well as patient preference. In practice, optimal management is guided by a multidisciplinary team where pharmacological, nonpharmacological, and surgical strategies can be simultaneously explored. This narrative review aims to provide an update on advanced therapies under investigation in clinical trials and offer insights into novel, nonpharmacological approaches with a focus on interventions entering late-phase trials that will be relevant to clinical practice in the near future.
INTRODUCTION
Despite the development of multiple biological therapies for Crohn’s disease (CD), remission rates at 1 year from individual agents are still only 30–50%. This decreases as second-line therapies are required and 80% of patients eventually require surgery in their lifetime.1 Early pharmacotherapies (such as anti-TNF) have a broad mechanism of action and are relatively ‘blunt’ tools used to dampen the dysregulated immune response in CD. As our understanding of the aetiopathogenesis of CD has improved, newer treatments have been developed targeting more specific pathways. Several nonpharmacological options, which can be used alongside pharmacotherapy, are also available or currently under investigation. This article focusses on novel therapies entering late-phase clinical trials with the aim of providing an overview of those therapies that are likely to be included in clinical practice shortly.
NOVEL PHARMACOLOGICAL THERAPIES
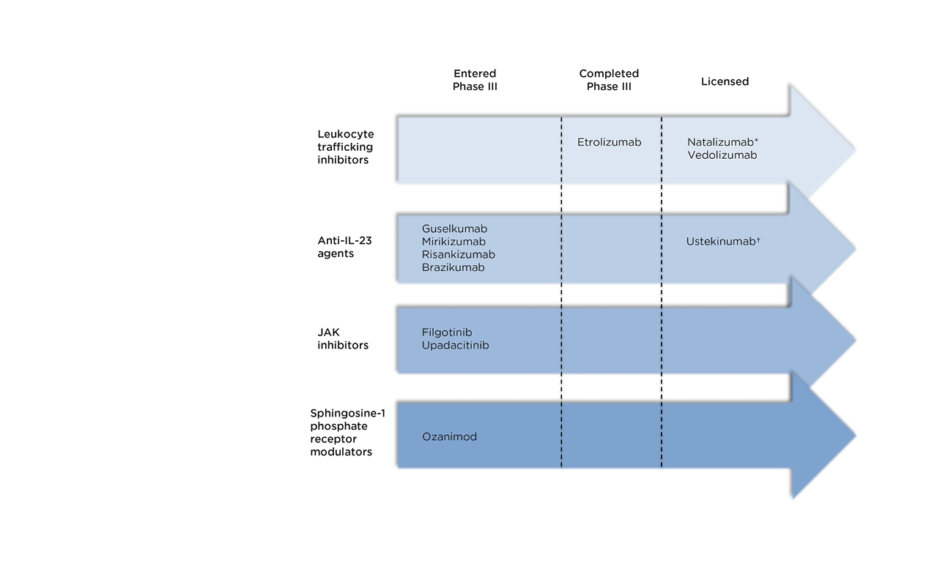
The therapies discussed below are summarised in Figure 1 according to their stage in clinical trials.
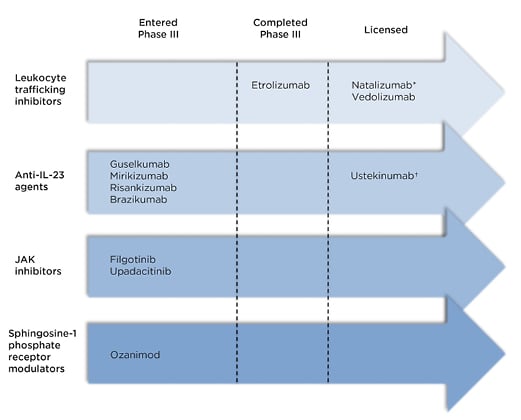
Figure 1: Recently licensed and late-stage pipeline drugs for the treatment of Crohn’s disease.
*Not licensed in Europe †Anti-IL-12 and IL-23
Anti-Adhesion Molecules
The recruitment of leukocytes to the vascular endothelium of inflamed tissue involves communication between integrins, cell adhesion molecules located on the surface of leukocytes, and endothelial adhesion molecules. Antagonism of this interaction limits the migration of leukocytes to the intestinal mucosa inhibiting intestinal inflammation.2 Vedolizumab was the first therapeutic agent in this class, but newer agents are showing promising results.
Etrolizumab targets the β7 subunit of both the α4β7 and the αEβ7 molecules. The Phase III BERGAMOT trial3 randomised 300 patients with moderate-to-severe CD to receive either placebo or subcutaneous etrolizumab (105 mg every 4 weeks or 210 mg at 0, 2, 4, 8, and 12 weeks) in a 1:2:2 ratio. Compared to placebo, both active arms had higher rates of clinical remission by Week 6 and endoscopic remission (ER) at Week 14 (105 mg: 21%, 210 mg: 17.4%, placebo: 3.4%). There were no differences between the placebo and active arms regarding adverse events (AE). Common AE included headache, fatigue, abdominal pain, and nasopharyngitis. There were no occurrences of progressive multifocal leukoencephalopathy.3 These promising results suggest a rapid effect and acceptable safety profile; the maintenance phase is currently underway.
IL-23 Antagonists
The IL-23 pathway has been associated with immune-mediated, chronic inflammatory diseases, including CD,4 and gene polymorphisms of the IL-23 receptor are associated with the development of CD.5,6 Blockade of IL-23 also has effects on IL-12 due to the shared p40 subunit, which is the mechanism of action for ustekinumab. However, animal models suggest that IL-12 has a beneficial role in infection prevention and immune response to cancer.7,8 Therefore, agents that selectively target the p19 subunit (unique to IL-23), may have an advantage over agents that target both IL-12 and IL-23, and appear to have similar side-effect profiles.4,9 Four such agents are in various stages of development. Guselkumab has been shown in case reports to be effective in the treatment of CD.10 The others are currently being investigated in randomised controlled trials (RCT).
In a Phase II RCT, mirikizumab led to higher rates of endoscopic response and remission, and clinical remission (CR) in patients with moderate-to-severe active CD.11 Patients were randomised to 200 mg, 600 mg, or 1,000 mg intravenous mirikizumab or placebo induction. At Week 12, endoscopic response and remission rates were significantly higher in the 600 mg (37.5% and 15.6%) and 1,000 mg (43.8% and 20.3%) groups compared to placebo (10.9% and 1.6%). This effect was mirrored with regards to CR; the maintenance phase is underway.
A recent Phase II trial investigating intravenous risankizumab induction in CD randomised patients to placebo, 200 mg, or 600 mg every 4 weeks. Higher rates of CR were noted amongst those in the 600 mg group (37%) versus placebo (15%).4 ER rates were higher at both doses of risankizumab (200 mg: 15%, 600 mg: 20%, placebo: 3%).
Notably, a near universal prior anti-TNF exposure rate was reported (93% exposed, 79% refractory). The open-label extension demonstrated Week 48 CR and ER rates of >70% and >50%, respectively.12
Similarly, brazikumab demonstrated a higher Week 8 clinical response rate compared to placebo (49.2% versus 26.7%; p=0.01). From a biomarker point of view, higher baseline serum concentrations of IL-22, a cytokine for which expression is induced by IL-23, were associated with greater likelihood of response.9
JAK Inhibitors
The downstream effects of inflammatory cytokines are often mediated by the intracellular JAK-signal transducer and activator of transcription signalling pathway. JAK inhibitors are small-molecule inhibitors of this pathway. Tofacitinib, a JAK1/3 inhibitor, has shown excellent response rates in ulcerative colitis that has not been replicated in CD (43.0% 10 mg tofacitinib twice daily versus 36.7% placebo; p=0.39).13 Reported side effects of JAK inhibitors include infections, viral reactivation (particularly herpes zoster), dyslipidaemia, and the potential for thromboembolic events.14-16 Two selective JAK1 inhibitors (filgotinib and upadacitinib) are currently under investigation.
The Phase II FITZROY study reported promising outcomes for filgotinib in moderately-to-severely active CD.17 At Week 10, CR in patients on 200 mg filgotinib was double that of placebo (47% versus 23%; p=0.008). No differences were noted in endoscopic outcomes, but the maintenance phase may yield better results. There were no significant differences in AE. One episode of herpes zoster reactivation and four serious infections (3%) occurred in the filgotinib arm versus none for placebo.
The Phase II CELEST study of upadacitinib in moderate-to-severe CD was recently published.18 Here, 220 patients were randomised to placebo or varying doses of upadacitinib. Significantly higher rates of ER were seen at the 24 mg doses (14–22%) compared with placebo (0%), though no difference in Week 16 CR rate was seen. There were three cases of herpes zoster reactivation, but none led to treatment discontinuation. No thromboembolic events were reported. Intestinal perforation was initially reported with tofacitinib, however, only two cases were noted in patients treated with upadacitinib, both occurring in regions of active luminal CD. Larger and longer-term studies will be needed to clarify whether this is causative or a result of lack of efficacy and thus progressive disease.
Selective inhibition of JAK1 appears to result in an increased anti-inflammatory effect with an improved safety profile (reduced rates of herpes zoster reactivation and thromboembolic events). This is because of reduced impact on haematopoietic cells and natural killer cells, which occurs with JAK2 and JAK3 inhibition.19
Sphingosine 1-Phosphate Receptor Modulators
Interactions within lymph nodes between the five different sphingosine 1-Phosphate (S1P) receptors and their ligands govern lymphocyte migration from lymph nodes into the circulatory system. By binding to S1P1 and S1P5 receptors, ozanimod causes the internalisation of these receptors limiting the emergence of B and T lymphocytes into the bloodstream and therefore, to inflamed intestinal tissue, preventing the propagation of inflammation.20
Recently, the STEPSTONE trial programme reported results from their open-label, uncontrolled Phase II trial.21 Patients with active disease received 1 mg ozanimod daily and underwent colonoscopy at Weeks 12 and 52 to assess endoscopic and clinical response. Score reductions of ≥25% and ≥50% in Simple Endoscopic Score for Crohn’s Disease (SES-CD) were seen in 43.3% and 26.7% of patients respectively, with larger reductions seen in those patients with less active baseline endoscopic disease. CR (Crohn’s Disease Activity Index [CDAI] decrease ≥100) and CR (CDAI <150) at Week 12 were seen in 66% and 46% of patients, respectively. Common side effects included nasopharyngitis, deranged liver function, arthralgia, rash, and hypertension. Rarely, nonselective agonism of S1P3 receptors may lead to bradycardia or macular oedema.22,23
Dual Biologic Therapy
Despite the advances noted above, patients with refractory disease are often not eligible for clinical trials. The use of dual biologic therapy is an emerging practice, often in patients where an additional agent is required to treat perianal disease or extraintestinal manifestations. Two case series have recently been published comprising 40 patients in total (32 with CD).24,25 Rates of clinical and endoscopic response were 41% versus 100% and 26% versus 93%, respectively. Nine out of 12 AE were infective in aetiology; none were serious despite a high frequency of concomitant steroid and immunomodulator use.
NONPHARMACOLOGICAL THERAPIES
Stem Cell Therapy
Luminal disease
Immune reconstitution therapies, such as stem cell transplantation (SCT), have generated attention for some time within inflammatory bowel disease (IBD) circles.26-29 Although reduction in CD activity was initially acknowledged as a collateral benefit of treatment for synchronous haematological disorders, the use of SCT as a treatment for CD has recently been explored. Following immunoablative conditioning, patients receive stem cells, either from a human leukocyte antigen (HLA) matched donor (allogeneic) or themselves, having been harvested prior to conditioning. Conceptually, allogeneic transplant resets the immune system at a genetic level, while autologous transplant replaces an aberrant immune milieu with uncommitted stem cells.28 The ideal result is complete resolution of inflammation, but failing that, a ‘resetting’ of therapeutic response to previously failed therapies is also a highly valuable outcome.26
ASTIC was an RCT of autologous stem cell transplant in patients with CD refractory to at least three immunosuppressive agents.28 Patients underwent transplant or standard of care, with the option of delayed transplant at 1 year. While the stringent primary outcome of treatment-free sustained remission at 1 year was not met (defined as CDAI <150, no use of corticosteroids or immunosuppressive drugs for the preceding 3 months, and no endoscopic or radiological evidence of active [erosive] disease anywhere in the gastrointestinal tract), more transplanted patients required no active treatment and had improved clinical and endoscopic disease activity than standard of care. In the subsequent pooled analysis of all patients who underwent transplant, 38% met the conventional endpoint of 3-month steroid-free CR, with 50% achieving complete endoscopic healing.29 However, the risks associated with transplantation are significant such that the trial was prematurely discontinued, with one death as a result of sinusoidal obstructive syndrome, along with a high rate of serious AE (34 in 13 transplanted patients versus five in four control patients in the 100 days following conditioning and transplantation; median difference: 1 [0–2] more serious AE; p=0.02).28 As a result, the ASTIClite study26 was designed to assess the safety and effectiveness of autologous stem cell transplant using a lower intensity mobilisation and conditioning regimen. Unfortunately, this trial has also been discontinued as a result of safety concerns.26 Accordingly, these safety concerns currently relegate SCT as a last-line treatment in refractory disease where the potential benefit is balanced against the considerable risks.
Perianal disease
Perianal disease is a common and debilitating phenotype of CD with disappointing long-term response rates to current therapies.30 This unmet need has necessitated an ongoing search for novel therapeutic approaches. The use of mesenchymal stem cells for treatment of perianal CD was first described in 2003.31 Adipose tissue-derived cellular aspirates are known to contain pluripotent stem cells capable of myogenic, chondrogenic, and adipogenic differentiation.32,33 Furthermore, mesenchymal stem cells show immunomodulatory properties, migrating to sites of active inflammation and secreting anti-inflammatory cytokines and upregulating CD4+ regulatory T cells (Treg).34 Multiple clinical studies using varying protocols have shown promise.35-38 In a Phase III placebo-controlled RCT, a significantly greater proportion of stem cell (darvadstrocel)-treated patients achieved clinical and radiographic fistula healing at Week 24 (50% versus 34%; p=0.024) with a favourable safety profile.35 The treatment showed durability and clinical and radiographic healing occurred in 56.3% of treated patients versus 38.6% controls (p=0.01) at 1 year.36 This resulted in the European approval of darvadstrocel, and a second, similar study is currently underway in the USA (ADMIRE-CD II). A European registry (INSPIRE) has been established to capture real-world effectiveness and safety.39 Currently, cost remains a significant barrier to treatment uptake.
Regulatory T Cell-Based Therapies
There is increasing appreciation of the notion that IBD is a disease of immune imbalance, with excessive inflammatory stimuli relative to insufficient numbers or functioning of down-regulatory immune mediators.40 Treg primarily function to control self-tolerance, tissue inflammation, and long-term immune homeostasis by exerting inhibitory effects on effector Treg.41 Animal models have highlighted their integral role in protecting against intestinal inflammation specifically,42 with transfer of Treg into mouse models of colitis resulting in amelioration of disease.43 In humans, loss of immune homeostasis as a result of quantitative and qualitative deficiencies in Treg is recognised as a driver of inflammation in CD.40,41 As a result, there is growing interest in the therapeutic potential of transferring healthy Treg into patients with IBD, as has been shown to be feasible with reassuring safety data in other immune mediated diseases.41
The effectiveness of Treg in IBD is thought to relate to their ability to home to the inflamed gut.43 This, in itself, presents a challenge given that gut inflammation does not necessarily produce a specific antigenic target. A Phase I/IIa study using Treg specific for ovalbumin (a food antigen) in 20 patients with active CD showed promise, with at least 40% experiencing a clinical response.44 A placebo-controlled trial of retinoic acid receptor-α-treated T-regs in CD is planned in the UK (TRIBUTE) with retinoic acid receptor-α having been shown to induce integrin α4β7 expression by Treg, potentially improving gut trafficking.41
Diet
In conjunction with a rapid growth in interest among patients,45 there is increasing scientific and medical recognition of the role of diet in the pathogenesis and treatment of IBD. Postulated mechanisms by which dietary manipulation may be beneficial include microbial modulation as well as effects on intestinal cell tight junctions and the mucous membrane.46,47 Exclusive enteral nutrition (EEN), consisting of the provision of a patient’s entire nutritional requirements through a liquid formula diet, has been demonstrated to be superior to corticosteroids in the induction of remission in paediatric CD.48-50 Despite issues with acceptability of EEN amongst adults, it has an established role in preoperative optimisation of patients with CD to reduce risk of complications,51 and there is evidence to supports its use in the setting of a new CD diagnosis.52,53
The CD exclusion diet (CDED) plus partial enteral nutrition (PEN) has been compared to EEN in a paediatric RCT, showing better tolerability and equivalent Week 6 corticosteroid-free remission.54 In the following 6 weeks, CDED plus PEN maintained remission while transfer to a free diet plus PEN did not. The CDED involved daily consumption of lean protein, starches, and fibres to act as substrates for short-chain fatty acid (SCFA)-producing bacteria. SCFA, such as butyrate, act as energy sources for colonic epithelium, perhaps explaining why low levels of SCFA may be related to IBD pathogenesis.47 The diet also involves exclusion of foods hypothesised to contribute to dysbiosis and negatively affect intestinal barrier and immune function.
Faecal Microbiota Transplantation
Faecal microbiota transplantation (FMT) has been shown to be a potential therapeutic option for induction of remission in ulcerative colitis. A recent systematic review of four RCT showed superior pooled remission rates compared with placebo.55 In CD, a pilot RCT randomised 24 patients, who had achieved steroid-induced CR to receive FMT or placebo via colonoscopy.56 A reduction in Crohn’s Disease Endoscopic Index of Severity (CDEIS) at Week 6 was noted in the FMT group (p=0.03). CD flare rates were numerically higher in the placebo group. Alpha diversity improved in the FMT cohort, but this was transient with normalisation at Week 14. This is consistent with earlier studies which suggested a ‘wearing off’ effect over time and a reported clinical benefit with sequential FMT therapies.57,58 A number of studies have also been limited by AE or disease flares.59 Given the heterogeneity amongst patients with CD, it seems that FMT may only benefit a proportion. However, further well-designed prospective studies in carefully selected patients are warranted to further elucidate its therapeutic role.
SURGERY
The lifetime risk of surgery for CD remains at about 80%. Five years postoperatively, 45% of patients have clinical postoperative recurrence (POR) and 20% have POR requiring further surgery. Only 22% have complete mucosal normality at 18 months.60 Despite the increased use of biologics over the last decade, data remain conflicting as to whether this has reduced the need for surgery.61,62
Novel Surgical Techniques
The European Crohn’s and Colitis Organisation (ECCO) guidelines recommend a stapled ileocolic side-to-side anastomosis, which is associated with fewer postoperative complications than a hand-sewn end-to-end technique.63 However, conflicting data exist regarding rates of POR amongst these anastomotic constructions.64,65 Novel approaches to reduce POR focus on the timing of surgery and exclusion or excision of the mesentery when constructing an anastomosis. Although mesenteric resection has been discussed for decades, uptake of this approach has been low due to the technical difficulties of safely disentangling an inflammatory mass without perforation, and achieving haemostasis whilst dividing a hypervascular and hypertrophied mesentery.66
A retrospective cohort (n=64) demonstrated that extensive mesenteric resection versus conventional ileo-colic resection (with limited mesenteric excision) was associated with a reduced likelihood of subsequent surgery (2.9%; n=1/34 versus 40%; n=12/30; p=0.003) and a shorter (but statistically insignificant) resection length. Here, 92% of reoperations in both cohorts occurred within 2 years. Furthermore, 10% (n=3) of cases in the conventional cohort (versus 0% undergoing extensive excision) required a third operation resulting in a cumulative reoperation rate of 40%. The degree of macroscopic fat wrapping, assessed intraoperatively, was associated with sites of the most severe mucosal disease, clinical disease severity, and risk of surgical recurrence.66 Further studies are under way to provide higher grade evidence with regard to the efficacy of this technique.67,68
The Kono-S (large lumen, hand-sewn anti-mesenteric functional end-to-end anastomosis) (Figure 2) was first performed in 2003 and has also been shown to reduce POR. A retrospective cohort study between 2003 and 2013 included 208 patients. Average Rutgeerts score was 2 at 5 years with no anastomotic surgical recurrence at 10 years. Additionally, 49% received postoperative infliximab but this did not significantly affect the surgical POR rate.69 This led to the SupREMe-CD Study, an RCT comparing the Kono-S with conventional stapled ileocolic side-to-side anastomosis. Patients were enrolled between 2016 and 2019 (n=83) with primary or recurrent CD. All patients received 3 months of postoperative metronidazole (as tolerated) and medical prophylaxis (at clinical discretion as per their perceived recurrence risk). On multivariate analysis, the only variable that significantly contributed to disease recurrence was the type of anastomosis. Kono-S resulted in reduced endoscopic recurrence (of any grade) at 1 year (22% versus 62%, p=0.001; reduced Rutgeerts score ≥3, 13% versus 35%, p=0.001) and delayed time to clinical recurrence (hazard ratio: 0.37, p<0.001).70 A similar USA-based trial is still recruiting, although interestingly, the design has no postoperative prophylaxis and assesses endoscopic recurrence at 3 months.71 Results from longer-term follow-up of these trials clearly has the potential to change clinical practice.
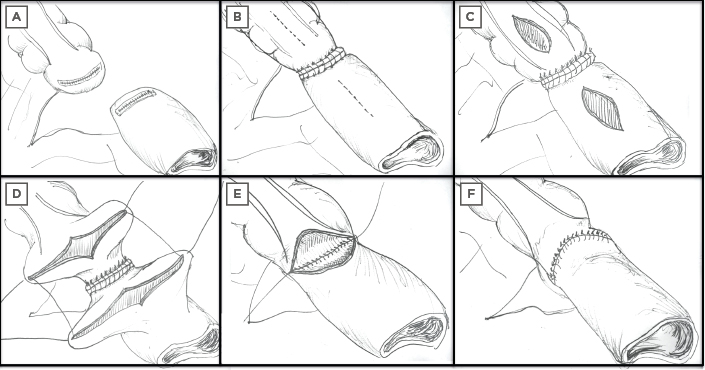
Figure 2: Steps involved in the construction of a Kono-S anastomosis. The mesentery of the resected segment remains in situ but is excluded from the anastomosis with a supporting column to prevent anastomotic distortion. A) Resection margins, B) supporting column created at the posterior/mesenteric border, C) new enterotomy for primary anastomosis to anterior/antimesenteric border, D–F) suturing and formation of primary Kono-S anastomosis.
As compelling as these data are, the pathophysiology that underlies the apparent benefit of the Kono-S remains elusive and it is unclear whether mesenteric inflammation drives mucosal inflammation or if it is a secondary phenomenon. It has been hypothesised that extensive mesenteric excision leads to greater lymph node resection, reducing inflammatory cell signalling and trafficking. Another hypothesis is that there may be mesenteric-dependent and independent CD phenotypes.72 Perhaps the techniques above lead to a ‘resetting’ of the complicated bidirectional signalling pathways, described by Li et al.,73 such that an anti-inflammatory state subsequently predominates.
Positioning of Surgery in Treatment Algorithms
The timing and indication for surgery is also critical. Long-term follow up data from the LIR!C trial have recently been presented.74 Early laparoscopic ileocolic resection was compared with infliximab in patients with inflammatory ileocaecal CD failing conventional treatment (steroids or immunomodulation). There was no difference in the duration of effect of the initial strategy, but the use of prophylactic immunomodulation postoperatively significantly decreased the risk for additional treatment or surgery in either arm. Five years postresection, 22% were off all treatment, 52% were taking immunomodulators (prophylactic or step-up) or required steroids, and only 26% required biologic therapy. In the infliximab arm, 50% proceeded to surgery, 38% continued TNF therapy, and 14% required additional treatment (with or without continued TNF therapy). Primary surgery has already been shown to result in equivalent quality of life scores in the short term; if the chance of surgical recurrence remains negligible beyond 10 years with still only a quarter requiring biologic therapy, then surgery may become a favourable first line option in the future.74,75
CONCLUSION
Several novel strategies in late phase clinical trials are showing promising results. From a pharmacological perspective, both risankizumab and upadacitinib will be available shortly and for those not eligible for clinical trials the use of dual biologic therapy may increase. It is likely that a significant proportion of patients will experience loss of response and need to explore alternatives. Harnessing the host’s immune response with cellular therapies is an exciting and complex field. However, it is not without risk, and at present, they remain reserved for patients with severe and refractory disease who have few, or no other viable options.
The application of stem cells for perianal disease, however, has a much more favourable risk–benefit profile, and should ongoing studies confirm efficacy, they may fundamentally alter perianal disease management pathways. Expansion of Treg cells ex vivo remains a challenge41 which will need to be overcome before larger trials can take place to investigate efficacy. In comparison to cellular therapies, dietetic interventions offer a low-risk option, but higher-grade evidence is required, and it is likely that these therapies will continue to be used alongside conventional therapy or in patients who are averse to immunosuppression.
Novel surgical techniques offer the potential to avoid repeated resection. Even more compelling, may be to combine the treatments described above and perform early laparoscopic ileocolic resection in inflammatory ileocaecal CD with either a Kono-S anastomosis or extensive mesenteric excision. Bearing this in mind, one may reasonably expect even higher rates of treatment-free remission, with time to POR potentially extending beyond a decade.