Abstract
Proton pump inhibitors (PPI) are one of the most widely prescribed drugs worldwide. They are the mainstay for treatment of most gastric acid-related disorders. PPIs are often used for inappropriate indications and unnecessarily prolonged durations. Initially thought to be a very safe class of drugs, concerns have been raised with regard to an increased risk of adverse events thought to be related to the long-term use of PPIs. PPIs are now known to be associated with increased risk of osteoporotic fractures, nutritional deficiencies (vitamin B12, magnesium, and iron), myocardial infarction, Clostridium difficile infection, community-acquired pneumonia, and gastric neoplasia. More recent evidence has shown that PPI use is also associated with renal impairment and dementia. Although these associations do not necessarily imply a causal link, PPIs should be used for the correct indications and for an appropriate duration. Prolonged use should be discouraged unless the benefits of treatment clearly outweigh the associated risks. More studies are needed to further explore these associations and to establish causality if present.
INTRODUCTION
Proton pump inhibitors (PPI) were introduced in the late 1980s and have since revolutionised the medical treatment of gastric acid-related disorders. Currently they are one of the most widely prescribed classes of drugs worldwide.1 PPIs are very potent gastric acid-suppressing agents and have largely superseded the use of histamine-2 receptor antagonists (H2RA). PPIs are indicated to treat conditions such as peptic ulcer disease, gastro-oesophageal reflux disease, erosive oesophagitis, Zollinger–Ellison syndrome, Barrett’s oesophagus, upper gastrointestinal bleeding, and as part of the therapy for the eradication of Helicobacter pylori infection.
PPI use has been increasing over the years and concerns have been raised regarding over-prescription in primary care and in-hospital settings,2,3 with 25–70% of PPI prescriptions found to be inappropriate. It is also common for the duration of use to be prolonged for longer than necessary once started.4
Numerous concerns have been raised over the potential long-term adverse effects of using PPIs. PPI use has been associated with increased fractures, malabsorption of nutrients, impaired cardiovascular health, infections, increased cancer risk, renal impairment, and most recently increased incidence of dementia and chronic kidney disease. The aim of this article is to discuss some of these important concerns by means of reviewing the current evidence.
PROTON PUMP INHIBITORS AND BONE HEALTH
Several epidemiological studies have demonstrated an association between PPI therapy and fractures, both osteoporotic and non-osteoporotic. Proposed theories for this association include reduced absorption of calcium, secondary to suppression of gastric acid secretion by PPIs. As calcium carbonate dissolution is pH-dependent, the dissolution and potentially absorbable form of calcium decreases with rising luminal pH. Suppression of gastric acid secretion also results in hypergastrinaemia which may cause calcium malabsorption and negatively influence bone metabolism, in part through induction of hyperparathyroidism. Excess parathyroid hormone (PTH) causes excessive bone remodelling where the rate of bone resorption exceeds the rate of bone formation.5
In animal models, 5 weeks of omeprazole administration led to hyperplasia and hypertrophy of the parathyroid gland, as well as increased PTH gene expression in chickens.6,7 These changes were coupled with reduced femur density.7 The same mechanism may also be responsible for the osteopenia observed in young rats receiving long-term PPI.8 In another study involving rats which had undergone total gastrectomy, administration of rabeprazole was not an exacerbating factor in bone metabolic disorders.9
The only human study to assess the effect of PPI on PTH levels was performed in Japan in 1993.10 Serum PTH levels and other markers of bone turnover, including serum osteocalcin, alkaline phosphatase, and tartrate-resistant acid phosphatase, increased after 8 weeks of omeprazole therapy. However, several issues were identified; for example, PTH was measured at only a single time point, which may not represent the dynamic effect of PTH over 24 hours. Secondly, the dose of omeprazole was 20 mg daily, and the effect of such a small dose on gastric acid secretion and gastrin is uncertain. Patients were also maintained on 500–700 mg of calcium intake daily that may have affected PTH levels. Despite these limitations, this study provided preliminary evidence supporting the effect of PPI on PTH and bone therapy in humans.
A meta-analysis encompassing 178,686 subjects showed no significant association between PPI therapy and bone mineral density.11 Another study involving 7,720 participants also found no association between PPI therapy and bone mineral density loss over time.12 A meta-analysis of 1,668 studies involving 223,210 fracture cases found a modest association between PPI use and increased risk of hip and vertebral fractures, but no evidence of duration effect in subgroup analysis.13 When stratified by duration of exposure, short-term use of PPI was associated with increased risk of hip fractures, whereas there was no significant increase in the risk of hip fractures with long-term PPI use. The authors advised interpreting their results with caution, adding that observational studies cannot clarify whether the association is a causal effect or a result of unmeasured/residual confounders.
PROTON PUMP INHIBITORS AND NUTRITIONAL DEFICIENCY: VITAMIN B12, MAGNESIUM, AND IRON
Gastric acid is needed for the absorption of protein-bound vitamin B12 from food, and is required for pancreatic proteases to cleave vitamin B12 from the protein, enabling its re-association with intrinsic factor and absorption in the terminal ileum.14
In a 2010 study of 36 institutionalised patients aged 60–80 years, chronic PPI users had lower serum B12 levels at baseline.15 In another study involving 250 elderly patients, no significant difference was observed in mean vitamin B12 levels between the long-term PPI users and those who did not take PPI.16 In a cross-sectional study of 659 adults aged 60–102 years, H2RA use did not influence serum B12 levels but PPI use was associated with diminished serum B12 levels.17
There have been several case reports describing the association of PPI use with hypomagnesaemia.18,19 The suggested hypothesis is that modifications in intestinal luminal pH due to PPI use reduce the active transport of magnesium via an alteration in the affinity of the transient receptor potential melastatin protein channels for magnesium.20 A meta-analysis of nine studies involving 115,455 patients found statistical significance between PPI use and the risk of hypomagnesaemia,21 even after adjusting for confounders. However, the authors could not reach a definitive conclusion due to significant heterogeneity among the included studies.
Gastric acid markedly improves the absorption of non-haem iron, therefore it is biologically plausible for PPI to reduce iron absorption. In one case series, two patients with anaemia failed to respond to oral iron therapy whilst on PPI, however their iron levels improved after withdrawal of PPI therapy.22 In a study of seven patients with hereditary haemochromatosis, long-term PPI use was associated with a significant reduction in the annual volume of blood removed during venesection.23
PROTON PUMP INHIBITORS AND CARDIOVASCULAR EVENTS
Initial concerns regarding adverse cardiovascular outcome associated with PPI use was attributed to drug-drug interaction with the antiplatelet agent clopidogrel. PPIs impair the bio-activation of clopidogrel by competitively binding to the hepatic cytochrome P-450 CYP2C19 in ex vivo and in vitro studies.24 Several retrospective observational studies have also reported an increased incidence of cardiac events when PPIs are used concomitantly with clopidogrel in patients with coronary artery disease.25,26
Other observational studies reported an increased incidence of adverse cardiovascular events with the use of PPI in patients who have had a myocardial infarction or have undergone percutaneous coronary intervention independent of clopidogrel usage.27-29 It was also noted that the association between adverse cardiac events and the use of PPI in patients already on clopidogrel persisted in patients taking all types of PPIs.30 Apart from the aforementioned studies involving patients with an increased baseline cardiovascular risk, several large population-based observational studies examining the risk of developing a new onset myocardial infarction in patients using PPIs in the general population showed that PPI users have an increased risk, independent of age and clopidogrel use.31,32
Results from COGENT, a randomised controlled trial measuring the efficacy and safety of concomitant administration of omeprazole with clopidogrel in patients with coronary artery disease, showed no significant difference in the primary cardiovascular outcome between the intervention and control groups. This study also recorded no difference in non-gastrointestinal bleeding events, which is interpreted as proof that PPIs do not diminish the antiplatelet effect of clopidogrel. However, this study was terminated prematurely when the sponsor lost its financing.33
In addition to the interaction with antiplatelet agents, several other mechanisms have been suggested to explain the association between PPI use and adverse cardiovascular events. Evidence from animal studies have shown that PPIs interfere with vascular homeostasis by reducing the nitric oxide (NO) synthase and endothelium derived NO levels, leading to endothelial dysfunction.30,34 Increased NO synthase activity is associated with decreased atherosclerosis progression in animal studies,30 whilst human epidemiological studies have shown that impaired NO synthase activity carries an increased cardiovascular risk.35 Experimental preclinical studies have suggested several possible ways by which PPIs may downregulate the NO production. PPIs may also reduce ascorbic acid and cobalamin levels, leading to increased degradation of NO. Additionally, PPIs have been known to reduce calcium and magnesium levels which may trigger cardiac arrhythmia.30
PROTON PUMP INHIBITORS AND INFECTIONS: CLOSTRIDIUM DIFFICILE AND PNEUMONIA
The use of PPIs has emerged as a significant risk factor for Clostridium difficile infection (CDI). Several meta-analyses have been published to address this topic. In one meta-analysis of 23 studies encompassing 300,000 patients, PPI use increased the incidence of CDI by 65%.36 Another meta-analysis of 42 studies that included 313,000 patients reported a probable association between PPI use and incidence of CDI.37 A third meta-analysis of 47 studies found a weak association between PPI use and risk of CDI in the presence of significant heterogeneity.38
The exact mechanism underlining this association is unclear. C. difficile is present in two forms: vegetative form which is sensitive to gastric acid, and spore form which is resistant to gastric acid. Gastric acid at a pH of <4 has potent bactericidal properties and acts as a natural defence against ingested pathogenic bacteria. The acid-suppressing effect of PPIs allows the vegetative form of C. difficile to survive in the stomach and possibly play a pathogenic role in the development of CDI.39 PPI use is associated with high levels of intra-gastric bile salt which could trigger spore germination in the stomach.38 In an in vitro study, there was an increased expression of C. difficile toxin A at a basic pH and this expression was increased further with PPI exposure.40 PPI use has also been shown to alter the gut microbiome, which predisposes to C. difficile infection.41,42
A systematic review of 33 studies and meta-analysis of 26 studies spanning over 20 years reported that outpatient use of PPI was found to confer a 1.5-fold increased risk of developing community-acquired pneumonia (CAP). PPI therapy was also associated with a 1.6-fold increased risk of hospitalisation for CAP, the risk of which was greatest during the first month of therapy (odds ratio: 2.1; 95% confidence interval: 1.39–3.16).43 These findings mirrored those of a previously published meta-analysis of studies which reported that PPI use showed increased risk of CAP, especially with short duration of use.44 Although the studies included within the meta-analysis were significantly heterogeneous, possible mechanisms to explain this observation include: micro-aspirations of altered gut microflora in the setting of increased pH due to PPI use, and a direct effect of PPIs causing pH dysregulation in the respiratory tract leading to an alteration of the respiratory microbiome.45 However, potential confounding by gastro-oesophageal reflux disease and protopathic bias limit the conclusions that can be drawn from these studies.
PROTON PUMP INHIBITORS AND GASTRIC NEOPLASIA
PPI use is known to cause fundic gland polyps, which typically regress with withdrawal of therapy.46 These polyps are generally benign with low risk for malignant transformation.47
Long-term PPI use in patients in the setting of H. pylori infection increases the risk of developing chronic atrophic gastritis, which is acknowledged as a risk factor for developing gastric cancer. There has been a change in the distribution of gastritis observed in this cohort of patients, where it gradually evolves to become a more corpus-predominant gastritis rather than being limited to the antrum.47 Patients with H. pylori infection with corpus-predominant gastritis are at high risk of developing gastric cancer.48 As PPI use promotes formation of atrophic corpus gastritis, their use in the setting of H. pylori infection could potentially increase the risk of gastric cancer. A causal link was demonstrated in animal studies; Mongolian gerbils exposed to omeprazole and H. pylori infection had a significantly increased incidence of atrophic corpus gastritis and gastric cancer.49 Similar findings have not been observed in human studies to date.
PPI use has also been implicated in the development of gastric carcinoid tumours. The potent acid suppression by PPIs can lead to increased gastrin secretion. Hypergastrinaemia causes hyperplasia of the enterochromaffin-like (ECL) cells in the stomach. The potent acid suppression induced by PPIs has been shown to trigger ECL cell hyperplasia, which has been shown to progress to gastric carcinoid tumours in animal studies.50 However, this finding has not been replicated in human studies, although there have been several case reports linking PPI use to ECL tumours.51 Assessment of safety data from two randomised studies, SOPRAN and LOTUS, evaluating regular PPI use in patients with gastro-oesophageal reflux disease for a 12-year and 5-year period, respectively, failed to confirm any PPI-induced gastric carcinoids.52
PROTON PUMP INHIBITORS AND RENAL IMPAIRMENT
A recently published observational study involving 10,482 patients followed-up for a median of 13.9 years showed that the use of PPIs was associated with a 20–50% increased risk of developing chronic kidney disease, after adjusting for confounding factors. This association was demonstrated only with the use of PPIs and was not seen in H2RA usage. Furthermore, twice daily dosing of PPI conferred a higher risk compared to once daily dosing. There was also a significant association between PPI use and incidence of acute kidney injury, suggesting that PPI use is an independent risk factor for both acute kidney injury and chronic kidney disease.53
Previously, it was understood that PPI use could lead to acute interstitial nephritis. This was first reported in 1992 and was thought to be an idiosyncratic reaction.54 Subsequently, several case-cohort studies have shown an increased risk of acute interstitial nephritis with PPI use.55,56 However, no causal link has yet been found to explain the significance of this association. The incidence of acute interstitial nephritis with PPI use might be under-diagnosed due to a low index of suspicion and could have been attributed to some other cause. Heightened awareness of this association by healthcare professionals has been recommended in the future to reduce possible misdiagnosis and to further our understanding of this association.57
PROTON PUMP INHIBITOR USE AND COGNITIVE IMPAIRMENT
A large scale longitudinal observational study published recently has examined the link between PPI use and the risk of developing dementia. Data for the years 2004–2011 was obtained from Germany’s largest statutory health insurer, covering one-third of the German population and nearly half of the elderly population. Analysis was performed on patients aged ≥75 years, and their exposure to the use of PPIs. Data from 73,679 patients were analysed, revealing a significantly increased risk of dementia with regular use of PPI after adjusting for multiple confounding factors.58 Previously it has been shown that chronic PPI use is associated with low vitamin B12 levels,59 and having low vitamin B12 levels in the elderly is associated with cognitive deficit.60 Despite having made a statistical association between PPI use and incidence of dementia, a causal link is yet to be established.
Several possible mechanisms have been suggested based on animal studies. Lansoprazole has been shown to increase amyloid beta (Aβ) protein production in both cell cultures and mice.61 Abnormal processing of the Aβ protein plays a pivotal role in the pathogenesis of Alzheimer’s disease, the most common form of dementia. In addition to blocking hydrogen–potassium ATPase in the gastric parietal cells, PPIs have also been shown to block vacuolar ATPase on the lysosomal membrane of inflammatory cells including macrophages.62 It has been postulated that PPIs decrease acidification of lysosomes in microglial cells (resident macrophages in central nervous system) and interfere with degradation of fibrillar Aβ protein, leading to increased Aβ protein levels in the brain.63
CONCLUSION
There is a growing body of evidence showing that long-term PPI use is associated with numerous potential adverse outcomes (Figure 1). Apart from the concerns regarding bone health and impaired nutritional status, an increased risk of adverse cardiac events has been attributed to the use of PPIs. The association with CDI is rather weak and the risk of developing CAP is short-lived and subject to possible protopathic bias. PPI use and risk of cancer is well-established in animal studies, however similar evidence in humans is lacking. More recently, the association of PPI use with renal impairment and dementia stem from large observational studies which lack the ability to establish a causal link; this is also the case in all other associated adverse outcomes. The absence of robust evidence from prospective studies to examine these associations is a challenge that needs to be addressed. Many of the studies from which these associations have been derived are prone to residual confounding factors and unidentified biases.
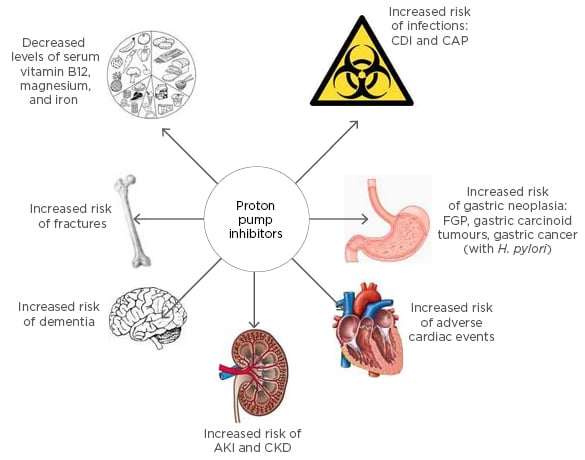
Figure 1: Adverse effects of long-term proton pump inhibitor use.
CDI: Clostridium difficile infection; CAP: community-acquired pneumonia; FGP: fundic gland polyps; H. pylori: Helicobacter pylori; AKI: acute kidney injury; CKD: chronic kidney disease.
We therefore recommend increasing the awareness of both primary and in-hospital healthcare professionals to promote judicious use of PPIs, curb over-prescription, and subsequently reduce the potential risk of developing PPI-related adverse outcomes. PPIs remain a useful group of drugs and should be used appropriately, but prolonged usage should be discouraged unless the benefits of treatment clearly outweigh the associated risks. Future research should be conducted to examine the associations between PPI use and adverse outcomes by means of rigorously designed studies.








