Abstract
Abdominal tuberculosis and its protean manifestations still create a diagnostic challenge for clinicians and remain an important concern in the developing world. Crohn’s disease, which is being increasingly recognised in countries where intestinal tuberculosis is prevalent, needs to be differentiated as the two diseases resemble each other in their clinical presentation, and in their radiological, endoscopic, and histological findings. New diagnostic modalities and scoring systems have facilitated the differentiation of Crohn’s disease from intestinal tuberculosis with good accuracy. Randomised trials have shown 6 months of therapy to be equivalent to longer durations of treatment for patients with abdominal tuberculosis. This review focusses on the recent advances in diagnosis and management of abdominal tuberculosis.
INTRODUCTION
Since its first description thousands of years ago, tuberculosis (TB) has ailed humanity and still haunts the human race. TB continues to be the top killer out of all infectious diseases worldwide, particularly in developing countries, according to the World Health Organization (WHO) global TB report in 2016.1 Extrapulmonary TB (EPTB) accounts for 15–20% of all cases and involvement of the abdomen is reported in 3.0–6.7% of EPTB cases.1-3 A recent report from India, which included 2,219 patients with EPTB, found that 11% of patients had infection with abdominal involvement; the abdomen was the third most common site after the lymph nodes and the pleura.4 Due to the insidious course of the disease, the nonspecific and protean manifestations of abdominal TB (ATB), and the difficulty in establishing the correct diagnosis, a clinician needs to have a high index of suspicion to reach a correct diagnosis.5 Poor socioeconomic status, undernutrition, poor hygiene, immunosuppression (such as HIV or AIDS), use of steroids or biologicals, history of a solid organ transplant (particularly renal transplant), and diabetes, among other factors, increase the risk of dissemination and occurrence of EPTB.5 ATB may be further classified as per the pattern of infection: luminal or intestinal, peritoneal, visceral (involving solid organs like the liver, pancreas, and spleen), or lymph nodal.6 The present review will discuss the recent advances in the field of ATB, predominantly focussing on intestinal and peritoneal TB.
ABDOMINAL TUBERCULOSIS
A number of mechanisms have been reported to result in causation of gastrointestinal TB, including spread through the haematogenous route from the primary pulmonary focus, ingested mycobacteria from the sputum produced from active lung lesions, direct or contiguous spread from adjacent organs, and through the lymphatics of infected lymph nodes.2,6 Peritoneal involvement is the most common form of ATB, seen in up to 58% of cases, followed by intestinal involvement in 40%.7,8
Clinical Presentation
As discussed, peritoneal TB is the most common presentation of ATB and accounts for 1.0–6.1% of all EPTB cases. Usually seen in young adults aged 20–40 years, peritoneal TB is more prevalent in women in developing countries, while in developed countries men are more commonly affected.9,10 The usual mode of spread is reactivation of latent foci in the peritoneum, seeding by a haematogenous route, often from distant pulmonary focusses and through ingestion of bacilli and infection of mesenteric lymph nodes. Alternatively, peritoneal TB can occur through contiguous spread from infected nodes, from ileocaecal TB, or directly from the fallopian tubes, stimulating latent foci reactivation.7,10
Three forms of peritoneal involvement are usually described; namely, the wet ascitic type, dry adhesive type, and fibrotic fixed type with loculated ascites and omental involvement.6 A rare presentation with overlap of the aforementioned three forms leads to adhesion and encapsulation of the bowel and an abdominal ‘cocoon’ formation; this distinct form of peritoneal TB presents with intestinal obstruction and mass per abdomen.11 The clinical presentation of peritoneal tuberculosis is of insidious onset, spanning over weeks to months, and the most common symptom is abdominal pain, seen in 49–100% of cases, followed by fever in 52–76%, weight loss in 61%, constipation in 7–31%, diarrhoea in ≤4.7%, and hepatosplenomegaly in 2–8%. Physical examination reveals ascites in 35–100% of patients, abdominal tenderness in 47%, and a doughy feel to the abdomen in ≤13%.7,9,10 Tubercular abdominal cocoon presents with intestinal obstruction in 73.3% and a lump in 60% of cases, and has previously been treated with surgical intervention, but a recent report describes a successful conservative management with anti-tubercular therapy (ATT) in the majority of the study cases.11,12
Intestinal TB (ITB) is grossly classified as ulcerative, hypertrophic, ulcerohypertrophic, and fibrotic (stricturing). The ileocaecal region is the most common location involved, affecting 44–93% of cases due to the relatively narrow lumen, stasis, and abundant lymphatics.5 The second most common location is the colon, while the stomach and oesophagus are rarely involved. Regardless of the site involved, presentation of abdominal pain, weight loss, fever, and features of intestinal obstruction are observed. Diarrhoea is uncommon but may occur with the ulcerative form of ITB.5,13-20 In addition to abdominal pain, colonic TB may cause rectal bleeding as one of the dominant symptoms, and occasionally the bleeding may be massive.6
Differential Diagnosis
The closest differential diagnosis of tuberculous peritonitis is peritoneal carcinomatosis. These conditions can be differentiated by ascitic fluid analysis, where cytology will be positive for malignant cells with high protein and low gradient ascites.21 Imaging can also help to differentiate, with computed tomography (CT) showing low attenuation mucinous ascites with amorphous calcifications scalloping the margins of the liver and spleen; however, no imaging finding is conclusive for discrimination of these entities.22 In patients presenting with intestinal obstruction, there is a possibility of extraluminal causes such as adhesions, masses (appendicitis, diverticulitis, peritoneal carcinomatosis, neuroendocrine tumour, lymphoma), strangulation, hernia, and malrotation. Alternatively, intraluminal causes like Crohn’s disease (CD), intussusception, radiation enteropathy, bezoars, and malignant masses need to be considered. Peritoneal carcinomatosis has similar imaging features like abdominal cocoon; namely, internal hernia, pseudomyxoma peritonei, peritoneal carcinomatosis, peritoneal mesothelioma, sclerosing malignant lymphoma, and malignant primary mesenteric tumours.23-25
Diagnosis of Abdominal Tuberculosis
The diagnosis of EPTB, especially ATB, is difficult to establish, with the primary reason for this being the low positivity of microbiological tests in this setting. Paustian’s criteria suggest that the diagnosis be established if any one of the following four criteria are observed: histology showing tubercles with caseating necrosis, suggestive operative findings and consistent histology from mesenteric lymph nodes, animal inoculation or culture showing growth of Mycobacterium tuberculosis, or histology showing acid-fast bacilli in the lesion.26 However, Paustian’s criteria are difficult to establish in most cases and Logan’s modification of the Paustian’s criteria, which uses response to ATT, has often been used to establish the diagnosis.27 On the other hand, the appropriate time and manner of establishing an adequate response to ATT has remained unclear. The investigational modalities for the diagnosis of ATB include radiological, biochemical, histology or cytology, and microbiological, including molecular tests and ancillary or supportive tests. Certain tests like chest roentgenogram and Mantoux skin test may have ancillary value but cannot be used as standalone diagnostic tools. Chest X-ray may be able to detect active or past evidence of pulmonary TB and thereby provide corroborative evidence of ATB; such changes may be detectable in a quarter of the patients.6 Mantoux test (tuberculin or purified protein derivative test), in particular, is compromised by false-positive (underlying Bacillus Calmette–Guérin vaccination, cross-reaction with other mycobacteria) and false-negative (disseminated TB, immunosuppression, recent infection, extremes of age). Interferon gamma release assays (IGRA) may overcome some limitations of Mantoux testing and do not have cross reactivity with Bacillus Calmette–Guérin or other mycobacteria; however, their positivity is consistent with M. tuberculosis infection but cannot be used to diagnose active disease.28 HIV testing should be performed in all patients suspected to have ATB.
INVESTIGATIONS
Radiological Evaluation
Radiological findings in patients with ATB may help in localising the site of involvement and directing further evaluation; however, no radiological finding is diagnostic of TB. Although barium studies were used frequently in the past, CT now provides the ability to identify intraluminal and extramural abnormalities and has replaced the use of barium studies.29 Findings supportive of a diagnosis include the presence of ascites, peritoneal thickening and enhancement, omental nodularity or thickening, lymphadenopathy, mural enhancement and thickening of the bowel wall, and intestinal strictures, as well as others (Figure 1). The presence of pulmonary lesions, a hypodense centre in an enlarged lymph node suggesting necrosis, and ascites are considered highly suggestive of TB (when discriminating from CD).30 CT enterography may have value over traditional contrast-enhanced CT, especially for better delineation of strictures.29 Tubercular strictures are usually short, smooth, and concentric; however, discrimination from other lesions, including fungal infections and malignant lesions of the peritoneal and intestine, requires histological evidence. A recent paper that compared the use of magnetic resonance enterography with small bowel follow-through suggested that magnetic resonance enterography diagnosed a higher number of strictures except when used in the evaluation of extraintestinal lesions.31
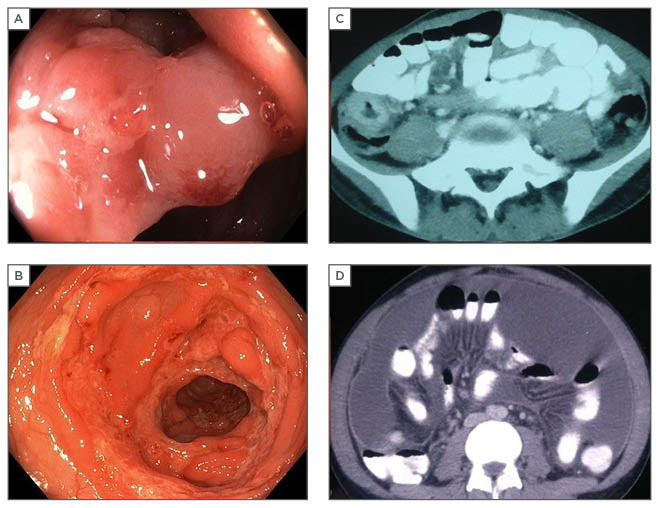
Figure 1: Radiological and endoscopic findings in abdominal tuberculosis.
A: colonoscopic image showing narrowed, thickened ileocaecal valve; B: circumferential colonic ulcers; C: thickened caecal wall on CT; D: CT showing ascites with peritoneal enhancement.
CT: computed tomography.
Diagnostic Evaluation for Peritoneal Tuberculosis
The utility of ascitic adenosine deaminase measurement for the diagnosis of peritoneal TB has been confirmed by systematic reviews.32,33 Adenosine deaminase is an enzyme secreted by activated lymphocytes and a value of >39 U/L in the ascites is indicative of a diagnosis of peritoneal TB.34 Since the positivity of microbiological tests, including smear for acid-fast bacilli and culture for TB, is exceedingly low, clinicians have to depend on the adenosine deaminase test to a large extent. However, the test may suffer from high false-negative rates, especially in the setting of underlying cirrhosis.35 Other findings on ascitic fluid analysis, which are consistent with a diagnosis of tubercular peritonitis, show a straw-coloured fluid, lymphocyte predominant cytology, high protein values, and low serum ascites albumin gradient.7 Sensitivity of polymerase chain reaction (PCR)-based tests is expected to be low and has been demonstrated by some studies; however, a positive test on peritoneal fluid is specific for the diagnosis.36 Needless to say, any diagnosis of peritoneal TB on the basis of this test must be established only after exclusion of other differential diagnoses, including peritoneal carcinomatosis by three cytological evaluations for malignant cells. Peritoneoscopy may need to be used in some cases and can show tubercles, thickened peritoneum, and adhesions.37
Diagnostic Evaluation for Intestinal Tuberculosis
Colonoscopy is the most important tool for evaluation of ITB as it helps in the characterisation of lesions, as well as in obtaining samples for microbiological and histological analysis. The colonoscopic findings in ITB include intestinal ulcers (usually transverse), pseudopolyps, strictures (usually short), and involvement of the ileocaecal valve (Figure 1). However, none of these are pathognomonic of TB and may be found in other conditions, including CD. The histological or cytological diagnosis is often based on fine-needle aspiration or biopsy obtained from radiology-guided sampling of the abdominal lymph nodes, peritoneal or omental thickening, or on endoscopic biopsies from the involved intestinal segments. The features suggestive of TB include the presence of granulomas, giant cells, caseating necrosis, and demonstration of acid-fast bacilli. The presence of granulomas is not unique to TB and may occur in other lesions, especially CD, fungal infections, and sarcoidosis; the presence of caseating necrosis is deemed to provide some degree of specificity to the diagnosis. Discrimination from CD is difficult, but the presence of multiple, large, confluent granulomas may be discriminative for TB. However, granulomas are only detected in a minority of cases (20–50%).38
Microbiological tools for the diagnosis include smear and culture for acid-fast bacilli and PCR-based tests; the low yield from the peritoneal fluid and intestinal biopsy samples is the Achilles’ heel of microbiological tests. The culture for TB and mycobacterium growth indicator tube (MGIT)-960 (Becton, Dickinson and Company, Franklin Lakes, New Jersey, USA) is unlikely to be positive in >50% of the cases.39,40 The use of PCR-based tests has been reported in multiple studies; a report on the use of multiplex PCR (using three probes: 16S rRNA, IS6110, and devR) provided excellent sensitivity for the diagnosis of both peritoneal and ITB but the findings await validation.41 Although the Xpert® MTB/Rif has emerged as an important tool for the diagnosis of pulmonary TB and some forms of EPTB (lymphadenitis), the reports on the use for ATB have indicated a limited benefit.42,43 In a report on the use of Xpert in peritoneal TB, the test was positive in only 4 out of 21 patients who were diagnosed with peritoneal TB.42 Similarly, in a report on ITB, only 3 out of 37 patients had a positive Xpert MTB/Rif test, suggesting that the sensitivity of the test for ATB would be low.43 In another study, the positivity of Xpert was reported to be lower than MGIT-960 for peritoneal TB (17.9% versus 25.5%). The yield of an in-house PCR (using three genes: hsp-66, esat-6, and ITS MAC) was also reported to be low.44 The bulk of evidence therefore suggests that, like other microbiological tools, PCR-based tests also provide a low sensitivity for the diagnosis of ATB.
A therapeutic trial of ATT can also be used in the diagnosis and discrimination of ATB from other conditions. A recent study from India has shown that endoscopic healing of ulcers in patients started on empirical ATT may allow differentiation of ITB from CD.45 While the global symptomatic response with ATT was 38% and 37% in patients with CD at 3 and 6 months, respectively, 94% and 99% of patients with ITB showed a response at 3 and 6 months, respectively. When endoscopic response was observed at the end of ATT, all ITB patients had mucosal healing, while only 5% of CD patients showed mucosal healing; similar findings were also documented in the validation cohort. Therefore, persistent symptoms after 3 months of ATT may indicate a diagnosis of CD; however, the presence of a clinical response to ATT does not exclude the possibility of CD and mucosal healing should be sought.45 Furthermore, a recent study has shown that a lack of decline in C-reactive protein levels in patients on treatment with ATT may suggest alternative diagnosis.46 Another concern in the management of ATB is drug resistance and, therefore, the knock-on effect in patients with HIV, those with a previous history of ATT, and those not improving on treatment should be considered and cultures for drug sensitivity must be done.
INTESTINAL TUBERCULOSIS OR CROHN’S DISEASE: HOW TO DIFFERENTIATE?
In countries where ITB is more prevalent, CD is being increasingly recognised.47-50 Both these chronic granulomatous disorders have similar clinical, endoscopic, radiologic, and histologic pictures; however, the natural history of both these disorders is strikingly different with serious implications regarding management. Misdiagnosis of one disease as another may be associated with multiple problems, including unnecessary immune suppression, drug toxicity, and delay in appropriate treatment. Table 1 shows important parameters used to differentiate CD from ITB.50-54 In one study, the presence of longitudinal or aphthous ulcers, anorectal lesions, and cobblestoning favoured CD, while transverse ulcers, patulous ileocaecal valve, <4 segments involved, and scars or pseudopolyps favoured ITB.55 Addition of CT enterography to colonoscopy increases the diagnostic accuracy and ability to differentiate CD from ITB from 66.7% to 95.2%. In a recent systematic meta-analysis of 38 studies, including 2,117 CD and 1,589 ITB patients, variables with significant odds ratios and low heterogeneity were selected to build a Bayesian model incorporating pre-test probability and diagnostic likelihood ratios to estimate probability of CD and ITB depending on local prevalence.56 Features favouring CD were reported to be male sex, blood in stools, perianal lesions, bowel obstruction, extraintestinal manifestations, longitudinal ulcers on colonoscopy, cobblestone pattern, stricture, mucosal bridging, and rectal involvement. Histology suggesting focally enhanced colitis and CT findings of asymmetrical mural thickening, mural stratification, comb sign, and proliferation of the fibrofatty tissue also indicate the presence of CD. The findings that favoured the diagnosis of ITB were pyrexia, night sweats, pulmonary involvement, ascites, transverse ulcers, patulous ileocaecal valve, and caecal involvement on colonoscopy. Histological findings of submucosal granulomas or confluent granulomas, lymphocyte cuffing, and histiocyte lined ulcers, CT findings of short segmental involvement, and a positive IGRA also favour ITB diagnosis.56
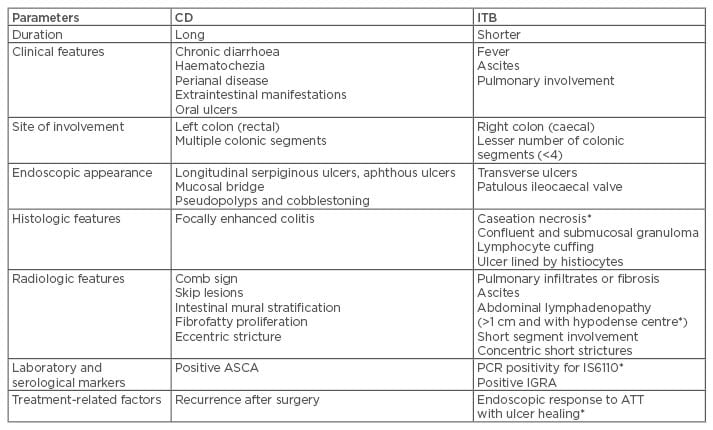
Table 1: Differences between Crohn’s disease and intestinal tuberculosis.
*Findings highly specific for diagnosis of ITB.
ASCA: anti-Sacchromyces cerevisae antibodies; ATT: anti-tubercular therapy; CD: Crohn’s disease; IGRA: interferon gamma release assay; ITB: intestinal tuberculosis; PCR: polymerase chain reaction.
Though differentiation has been validated in a local cohort from Bangkok, Thailand, with high diagnostic accuracy, the results need to be validated further in several local populations across countries with high prevalence of CD and ITB to prove its strength.56 A recent systematic review and meta-analysis of studies looking at the accuracy of CT features in differentiating ITB from CD involving six studies with 417 and 195 patients of CD and ITB, respectively, has shown that a comb sign and necrotic lymph nodes are features with the best diagnostic accuracy to differentiate CD and ITB.30 A further meta-analysis involving nine studies, with 340 CD and 369 ITB patients, has shown that PCR for M. tuberculosis has a high specificity for distinguishing ITB from CD; however, due to a very low sensitivity, a negative result does not completely rule out the diagnosis of ITB.57 Finally, a systematic review and meta-analysis involving 11 studies with 1,081 patients with CD or ITB has shown a high specificity of IGRA and anti-Saccharomyces cerevisiae antibody for the diagnosis of ITB, with a supplementary role of distinguishing ITB from CD.58 However, the results from the Indian studies are not encouraging. In addition, faecal TB PCR for IS6110 specific for M. tuberculosis may help discriminate ITB from CD.59
TREATMENT OF ABDOMINAL TUBERCULOSIS
Treatment of ATB, as with other forms of EPTB, is challenging. The challenges a clinician encounters include determining the appropriate duration of treatment, criteria to determine appropriate response to treatment, determining the end points of treatment, and recognition and treatment of sequel of ATB. The question regarding the appropriate duration of therapy has been addressed by multiple randomised trials, and a Cochrane review on the issue.60,61 The systematic review of the three included trials, involving 328 participants, suggests that 6 months of treatment (with isoniazid, rifampicin, pyrazinamide, and ethambutol) is adequate in patients with ATB (primarily intestinal and peritoneal).61 The included trials did not involve patients with HIV or comorbidities, or those who had received ATT previously as well as those with other forms of ATB (e.g., hepatic and pancreatic) and therefore the results may not be applicable to these patients.
The appropriate method of follow-up of patients with ATB is a challenging issue. Even though the disease may heal, persistence of symptoms may be related to sequelae like peritoneal adhesions or intestinal strictures. Continued symptoms may result in an unwarranted prolongation of treatment. Therefore, the follow-up should include both objective and subjective parameters for assessment of response. For ITB, demonstration of endoscopic healing (especially the ulcers) appears to be an excellent method to document response and may be performed at 2–3 months or later (Figure 2). A recent paper demonstrated the use of this approach to discriminate ITB from CD in patients where a therapeutic trial of ATT was administered in indeterminate lesions.45 In cases of non-healing ulcers, the possibility of drug-resistant TB or an alternative diagnosis must be considered; the frequency of drug-resistant TB varies from one geographic location to another but culture and drug sensitivity should be performed in patients with non-healing mucosal lesions or at the initial evaluation in those with previous history of ATT therapy or patients with HIV.62 While an important concern in western India, drug-resistant TB is uncommon in patients with ATB reported in north India and South Korea.43,62,63 For the follow-up of patients with peritoneal TB, abdominal ultrasonography to look for resolution of ascites could be an appropriate strategy (Figure 1). Other parameters that are often used to assess response include improvement in appetite and general wellbeing, defervesce of fever, and weight gain. A recent paper also suggests that patients with the special form of peritoneal TB, abdominal cocoon, may also benefit from a conservative approach with ATT and the majority of patients can therefore avoid surgery.11 In a recent multicentre Indian study, the treatment completion rates for ATB were lower than most other forms of EPTB (like pleural, lymph-nodal, genitourinary) although the reasons for this are not clear.4
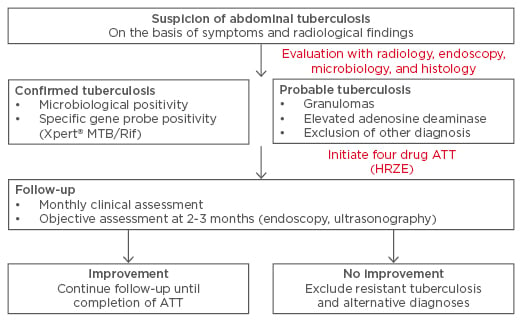
Figure 2: Suggested follow-up of patients with abdominal tuberculosis.
ATT: anti-tubercular therapy; HRZE: isoniazid, rifampicin, pyrazinamide, and ethambutol.
A recent study noted that while symptom resolution occurs in half of the patients, stricture resolution was noted only in a quarter of the patients. Furthermore, colonic stricture was more likely to persist and therefore endoscopic or surgical treatment may be needed for symptomatic patients.64 Possible reasons for surgical intervention could include perforation, gastrointestinal bleeding, unremitting or recurrent intestinal obstruction due to intestinal strictures, adhesions, and cocoon, as well as others. In a recent series of 756 patients with ATB seen over a period of 20 years, a third of the cases needed surgery; however, this may be a biased figure since the data are from a surgical unit.65 Other reports suggest that a subset of the patients will need surgery.66 Acute presentation in the form of intestinal perforation or unremitting intestinal obstruction warrants surgical intervention. However, in cases where the strictures are amenable to endoscopic dilatation (short, endoscopically reachable), endoscopic dilatation may help avoid surgery.67
CONCLUSION
To conclude, ATB remains an important concern in developing countries. The discrimination of ITB from CD is challenging and often necessitates a trial of ATT. ATT therapy for 6 months is usually adequate for mucosal healing and resolution of ascites, but sequelae like intestinal strictures may result in persistent symptoms needing endoscopic dilatation or surgical intervention.








