Abstract
Gastric cancer is the second most common cause of cancer-related deaths worldwide, and the 5-year overall survival rate for advanced gastric cancer is ≤25%. Metabolism is a critical process for maintaining growth and other functions in cancer cells; in these cells, the metabolic process shifts from oxidative phosphorylation to aerobic glycolysis and the expression of pyruvate kinase (PK) splice isoform M2 (PKM2) is upregulated. A PubMed search focussing on PK in gastric cancer was conducted and 32 articles were initially collected; 12 articles were subsequently excluded from this review. PKM2 is responsible for tumour growth and invasion and correlates with short survival times and cancer differentiation. Pyruvate dehydrogenase kinase 1 is associated with cell proliferation, lymph node metastasis, and invasion. Measurement of PKM2 or pyruvate dehydrogenase kinase 1 in the blood or stools could be a good marker for gastric cancer in combination with the glycoprotein CA72-4. The review arose from the need for new biomarkers in the management of gastric cancer and had the primary objective of determining whether PK could be used as a marker to diagnose and monitor gastric cancer.
INTRODUCTION
Gastric cancer is the fourth most common cancer in men worldwide and the second most common cause of cancer-related deaths worldwide.1,2 In 2008, approximately 700,000 deaths were related to gastric cancer, equating to 10% of all cancer-related deaths, and 989,600 new cases of gastric cancer were diagnosed, representing 8% of all new cancer cases.1 The number of gastric cancer patients diagnosed in the early stage of the condition is increasing due to improvements in early diagnosis; however, many cases are still found in the advanced stage.1,2 For advanced gastric cancer, the 5-year overall survival rate is ≤25%.3 Therefore, identification of molecular therapeutic targets and novel biomarkers for early diagnosis and individualised therapy is of great importance.
Metabolism is a critical event in maintaining growth and other functions in cancer cells. Tumour cells require high metabolism rates to sustain active proliferation and other biological events that require a large amount of energy. In contrast with non-cancerous mammalian cells, cancer cells are always in a hypoxic environment due to their fast growth and the limited oxygen supply. The metabolism in tumour cells shifts from oxidative phosphorylation to aerobic glycolysis, known as the Warburg effect.4 For aerobic glycolysis, pyruvate kinase (PK) is a rate-limiting enzyme5,6 that catalyses dephosphorisation of phosphoenolpyruvate to pyruvate to yield one molecule of ATP. This molecule has four isoforms: PK type M1 (PKM1), PK type M2 (PKM2), PK type L, and PK type R; PKM1 is found in muscle and brain tissue, PKM2 in embryonic and tumour cells, PK type L in the liver and kidneys, and PK type R in red blood cells.
In tumour tissues, the expression of PKM2 is upregulated since it is essential for the process of aerobic glycolysis.5 Polypyrimidine tract-binding protein 1 (PTBP1) regulates alternative splicing of PK, resulting in PKM2 (and can lead to switching of the PKM isoform from PKM1 to PKM2), which is expressed in a broad range of human cancers.7 PKM2 expression was shown to be involved in early tumourigenesis,8 and an increase in PKM2 level correlates with tumour size and stage.9 This high expression indicates that active aerobic glycolysis occurs and regulates numerous cell functions in these cells.10 Moreover, PKM2 expression is strongly correlated with gastric cancer differentiation; differentiated types of cancers express more PKM2 protein than undifferentiated cancers.11 Clinical studies have demonstrated that PKM2 is released into the bloodstream and levels of PKM2 in ethylenediaminetetraacetic acid (EDTA) plasma samples are increased in gastrointestinal cancers.12
However, the clinical and prognostic implications of PKM2 as a marker for gastric cancer are still unclear. Abnormal glucose metabolism in cancers can be used as a target for cancer treatment. Several agents targeting glycolysis have been reported to have significant cytotoxicity for cancer cells in preclinical studies, with some of these agents having advanced into clinical studies.13 However, the use of these agents for the treatment of gastric cancer has not been reported. The objective of this literature-based review was to determine whether PK could be used as a marker to diagnose and monitor gastric cancer.
METHODS
A PubMed search was conducted focussing on PK in gastric cancer. The PubMed database was searched with the terms (“stomach neoplasms”[MeSH Terms] OR (“stomach”[All Fields] AND “neoplasms”[All Fields]) OR “stomach neoplasms”[All Fields] OR (“gastric” [All Fields] AND “cancers”[All Fields]) OR “gastric cancers” [All Fields]) AND ((“pyruvic acid”[MeSH Terms] OR (“pyruvic”[All Fields] AND “acid”[All Fields]) OR “pyruvic acid”[All Fields] OR “pyruvate”[All Fields] OR “pyruvates” [MeSH Terms] OR “pyruvates” [All Fields]) AND “phosphotransferases”[MeSH Terms])”.
A total of 32 articles were initially collected. Twelve articles were subsequently excluded from this review: 6 because the studied molecules were not PK, 2 because the studied tissues were not gastric tissue, 1 because the article was not related to gastric cancer, and 3 because they were not written in English. Ultimately, 20 studies were included in the analysis.
RESULTS
The tumour PKM2 isoform has been shown to be present not only in plasma but also in faeces, indicating that PKM2 may serve as a potential marker for screening colorectal and gastric cancers in high-risk individuals.14 The main results of the selected studies are listed in Table 1.12,15-32
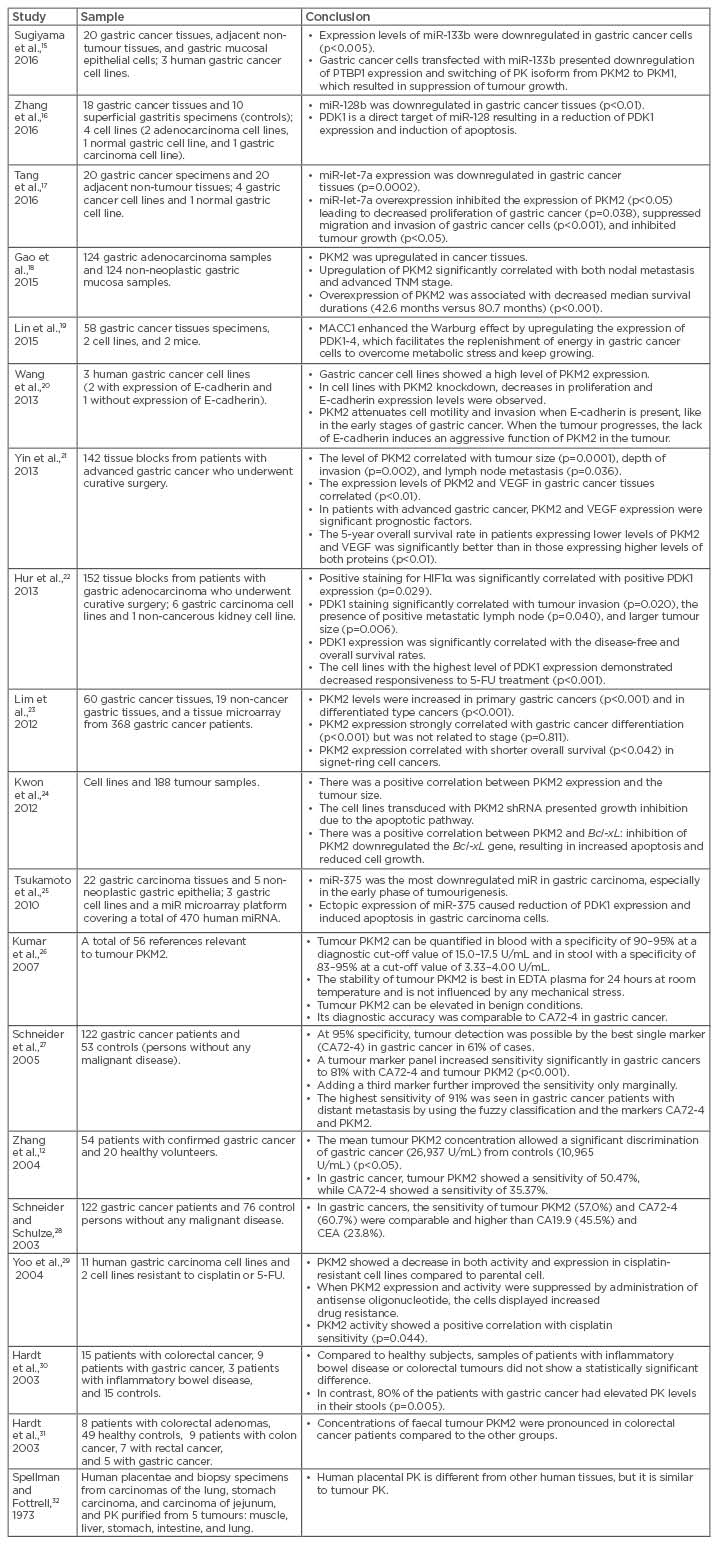
Table 1: The main results of selected studies.
CA: cancer antigen; CEA: carcinoembryonic antigen; EDTA: ethylenediaminetetraacetic acid; FU: fluorouracil; HIF1α: hypoxia-inducible factor 1-alpha; miR: microRNA; PDK: pyruvate dehydrogenase kinase; PK: pyruvate kinase; PKM1: pyruvate kinase isoform M1; PKM2: pyruvate kinase isoform M2; PTBP1: polypyrimidine tract-binding protein 1; shRNA: short hairpin RNA; TNM: tumour, node, metastasis; VEGF: vascular endothelial growth factor.
DISCUSSION
MicroRNA (miRNA) are small non-coding RNA molecules, 18–25 nucleotides in length, that bind to the 3’ region of target messenger RNA and induce silencing of protein expression.33-35 miRNA play important roles in a variety of processes, such as cell development, apoptosis, and cell proliferation.33,34,36 Dysregulation of miRNA is involved in many diseases and most miRNA modulate tumour suppressor genes in various types of cancers.33,34
miR-133b was initially considered to be a muscle-specific miRNA involved in the development of skeletal muscle, myoblast differentiation, and myogenic-related diseases; however, a wider expression of miR-133b was found in diverse tissues.37 miR-133b plays an important role in non-muscle-related disease, such as Parkinson’s disease, cardiac failure, and cancer;38 expression of miR-133b is downregulated in many types of cancers.39
Sugiyama et al.15 concluded that miR-133b was significantly downregulated in cell lines and in gastric cancer tumour tissues compared with normal cells and tissues, respectively. Furthermore, the ectopic expression of miR-133b markedly inhibited cell proliferation through the induction of autophagy. These findings indicate that miR-133b acts as a tumour suppressor miRNA through the perturbation of the Warburg effect in gastric cancer cells. The authors also proved that, in gastric cancer cell lines transfected with miR-133b, the expression of PTBP1 was markedly downregulated, the PKM isoform expression was switched from PKM2 to PKM1 for a short duration, and the tumour growth was suppressed. The authors suggested that PTBP1 could be a target molecule for the development of anti-cancer drugs.15
miR-128 includes miR-128a and miR-128b,40 and their aberrant expression was observed in many malignant tumours, but the function of miR-128b in gastric cancer is not yet known. Zhang et al.16 found that miR-128b was downregulated in gastric cancer tissues and cell lines, suggesting that it might negatively modulate the carcinoma progression. The results showed that overexpression of miR-128b decreased cell proliferation, inhibited cell viability by arresting them in G0 or G1 phase (the proportion increased by approximately 10%; p<0.05), suppressed invasion, and accelerated apoptosis (the rate increased 6.5–8.8-fold) through inactivation of the Akt/nuclear factor-κB (NF-κB) axis by targeting pyruvate dehydrogenase kinase 1 (PDK1).
miR-let-7a plays a role in cell differentiation, apoptosis, proliferation, and metabolism,41 and its levels are low in different human cancers; downregulation is associated with cancer aggressiveness.42 Tang et al.17 concluded that miR-let-7a was highly downregulated in gastric cancer tissues and cell lines, and its overexpression resulted in the significant decrease in cell proliferation rate, colony formation, migration, invasion, and tumourigenicity. Suppression of cell growth, migration, and invasion of gastric cancer cells was achieved by downregulating the expression of PKM2. Coexpression of PKM2 and miR-let-7a could rescue a tumour inhibited by miR-let-7a, indicating that PKM2 is the target of miR-let-7a in gastric cancer. Nevertheless, the specific mechanism by which miR-let-7a affects the expression of PKM2 was not clear. In addition, Gao et al.18 found that PKM2 was overexpressed in gastric cancer and expression correlated with nodal metastasis, advanced tumour, node, metastasis (TNM) stages, and poor prognosis.
E-cadherin plays a critical role in maintaining epithelial integrity, and the loss of E-cadherin affects the adhesive repertoire of a cell. This molecule is also a tumour suppressor with a frequently reduced or silenced expression, and its re-expression can induce morphologic reversion.43 Epidermal growth factor receptor (EGFR) proteins enhance cell motility and at least two distinct intracellular signalling pathways are required for EGFR-mediated cell motility: the pathways using phospholipase C-γ and the mitogen-activated protein kinase pathway.44 Wang et al.20 demonstrated that the knockdown of PKM2 decreased the activity of E-cadherin and enhanced the EGFR signalling pathway in the cell lines that were positive for E-cadherin expression. However, in the undifferentiated gastric carcinoma cell line, which lacked E-cadherin expression, PKM2 promoted cell migration and invasion.
The major factors that determine the prognosis of gastric cancer include lymph node metastasis, depth of tumour invasion, and tumour size. Tumour angiogenesis plays a critical role in metastisation and tumour growth. Any increase in a tumour mass must be preceded by an increase in the microvasculature to deliver nutrients and oxygen to the tumour and remove products of tumour metabolism. Without new blood vessels, most tumours would never grow beyond 1–2 mm in diameter and would remain localised to the primary site.45 EGFR is one of the most important regulators of angiogenesis. Yin et al.21 concluded that PKM2 and vascular endothelial growth factor expression were positively correlated with the prognosis of advanced gastric cancer.
Hur et al.22 concluded that glucose transporter-1 and PDK1 expression were significantly associated with tumour progression, although only PDK1 expression was an independent prognostic factor for patients who received 5-fluorouracil (FU) adjuvant treatment. Treatment with dichloroacetate, a PDK1 inhibitor, reduced lactate production and increased responsiveness to 5-FU in cell lines that expressed high levels of PDK1.
Lim et al.23 showed that PKM2 was overexpressed in gastric cancers both at the messenger RNA and protein levels compared to non-cancerous gastric tissues. Moderately and well-differentiated adenocarcinoma showed significantly higher expression of PKM2 (60.0% PKM2-positive cells) in contrast with signet-ring cell cancers, which showed 17.7% PKM2-positive cells. NF-κB is a transcription factor that controls the expression of proteins involved in the regulation of immune response and cell survival.46 Deregulation of NF-κB signalling is associated with oncogenesis and cancer malignancies because its activation increases the expression of many genes involved in cell proliferation, metastasis, angiogenesis, and anti-apoptosis pathways.47
Kwon et al.24 identified PKM2 as an overexpressed gene in gastric cancer patients at both the transcriptional and protein levels and showed that PKM2 expression level affected the survival of gastric cancer cells. High PKM2 expression was associated with poor prognosis in gastric cancer patients. PKM2-mediated NF-κB stabilisation may underlie the molecular basis for increased survival in gastric cancer cells, in part by regulating the expression of Bcl-xL, an apoptosis-related gene.
miR-375 has been reported to be downregulated in head and neck,48 pancreatic,49 and hepatocellular carcinomas,50 but its function in cancer remains to be determined. In gastric carcinoma cells, Akt phosphorylation has been reported to promote cell survival and act against apoptotic stimuli.51 Tsukamoto et al.25 found that miR-375 was the most downregulated miRNA from a microarray with 470 human miRNA. Re-expression of miR-375 in gastric carcinoma cell lines resulted in induction of apoptosis and reduced cell viability. Exogenous miR-375 suppresses the expression of PDK1, resulting in decreased phosphorylation of Akt in gastric carcinoma cells. Decreased expression of miR-375 may provide a survival advantage to gastric carcinomas via activation of the PDK1/Akt survival pathway. Downregulation of miR-375 results in enhanced expression of 14-3-3ζ and provides a survival advantage to gastric carcinoma.
Currently used tumour markers have a low sensitivity for detecting cancer and their role is limited to detecting recurrence after surgery or monitoring the response to treatment. Tumour PKM2 can be measured in the stool and in the bloodstream by a highly sensitive enzyme-linked immunosorbent assay (ELISA). Kumar et al.26 compared different tumour markers and concluded that the diagnostic accuracy of tumour PKM2 was comparable to CA72-4 in gastric cancer; using a combination of these tumour markers increased the diagnostic strength. Schneider et al.27 found that the sensitivity of tumour PKM2 (57%) was comparable to CA72-4 (61%) in gastric cancer, and the two-marker combination increased the sensitivity to 81% (p<0.001). For the discrimination of malignant versus non-malignant diseases, the fuzzy classificatory (a mathematical procedure for a non-invasive analytical method) increased sensitivity by 20% compared to the best single marker in gastric cancer.27 In another study, Schneider and Schulze28 demonstrated that the discrimination power of tumour PKM2 was superior in colorectal, gastric, and oesophageal cancers without distant metastasis, whereas Zhang et al.12 concluded that the sensitivity of tumour PKM2 in the diagnosis of gastric cancer was lower than that in the diagnosis of colorectal cancer, although it was higher than that of CA72-4. In addition, Hardt et al.30 found that stool samples of gastric cancer patients had elevated PK concentrations compared to healthy controls and inflammatory bowel disease patients. In another study by Hardt et al.,31 the authors found a significant difference in faecal tumour PKM2 concentrations between cancer patients and controls, and the highest concentrations were observed in colorectal cancer cases. Lastly, studies also showed that the sensitivity of faecal tumour PKM2 was 73.00% and the specificity was 78.00%;52 whereas the sensitivity and specificity of serum PKM2 in gastric cancer was 50.47% and 90.00%, respectively,12 and 66.70% and 88.90% in colorectal cancer, respectively.53
Chemotherapies for advanced gastric cancer, usually containing cisplatin and/or 5-FU, have response rates of 20–40%, with between 6 and 12 months of median survival.54 However, cancer cells can be unresponsive to drug treatment at the outset of therapy (intrinsic resistance) or they may become unresponsive after exposure to the chemotherapy agent (acquired resistance).55 Yoo et al.29 linked PKM2 activity and cisplatin-resistance mechanisms. They observed that cisplatin resistance correlated with decreased levels of PKM2 protein and activity in human gastric carcinoma cell lines and that lowering PKM2 expression through antisense transfection increased cisplatin resistance.
CONCLUSION
Measurement of PKM2 or PDK1 in the bloodstream or stools of patients could be a good marker for gastric cancer when analysed in combination with CA72-4; these markers are related to tumour burden, proliferation, invasion, differentiation, and lower survival. Once the diagnosis of gastric cancer is set, the combination of these two biomarkers could help monitor the response to treatment, as well as detect progression or relapse. PKM2 is also associated with poor prognosis, so patients with higher levels of PKM2 at diagnosis could have lower survival rates. A consideration for future development is that targeting PKM2 could become a new therapeutic approach for the treatment of gastric cancer patients.








