Abstract
The authors report the first case of oesophageal cytomegalovirus (CMV) and herpes simplex virus (HSV) co-infection in an immunocompetent patient with an associated oesophagal stricture. The authors also review the literature on oesophageal strictures related to CMV and/or HSV oesophagitis. CMV and HSV co-infection is well documented in immunocompromised patients. The oesophagus is one of several organs known to harbour co-infection. To the knowledge of the authors’, its association with an oesophageal stricture is rare and has only been noted in patients with HIV. The authors report a case of an immunocompetent 40-year-old woman with a past history of iron deficiency anaemia and idiopathic hypertension who presented with dysphagia. Investigations revealed a circumferential oesophageal stricture, with biopsies positive for CMV and HSV. This patient was HIV-negative and had no evidence of immunodeficiency. The patient was treated successfully with valganciclovir and multiple endoscopic oesophageal dilatations. This presentation in an immunocompetent patient has not been described in the literature to the authors’ knowledge and represents a valid differential diagnosis to be recognised in clinical practice.
Key Points
1. This is the first time an oesophageal stricture related to cytomegalovirus (CMV) and herpes simplex virus (HSV) co-infection in an immunocompetent individual has been described. All cases of previously described CMV-related oesophageal strictures have been in the context of immunosuppression related to HIV or medication-related immunosuppression.
2. This study is a case report with a review of the literature on CMV-related oesophageal strictures. The review includes seven studies involving 18 patients over 33 years from being first described in 1991 (up to 2024).
3. CMV and HSV chronic infection must be considered in clinical practice and screened for in patients presenting with dysphagia secondary to an oesophageal stricture in the absence of malignancy.
CASE PRESENTATION
A 40-year-old woman presents with 3 weeks of progressive dysphagia and 10 kg of associated weight loss. Gastroscopy was performed, demonstrating an intrinsic oesophageal stricture at 23 cm from the incisors, which was unable to be traversed by a slim gastroscope (5.4 mm outer diameter), as shown in Figure 1. A subsequent CT scan revealed concentric wall thickening of the mid-thoracic oesophagus, raising concerns of oesophageal malignancy, also shown in Figure 1. Endoscopic biopsy was performed on the stricture revealing cytomegalovirus (CMV) and herpes simplex virus (HSV) oesophagitis with no dysplasia and no evidence of malignancy. Past medical history included iron deficiency anaemia and idiopathic hypertension, treated with amlodipine-valsartan 5-160 mg once daily. There was no history of proton pump inhibitor or significant antibiotic use. An extensive history was taken from the patient to determine the cause of immunosuppression. Most notably, there were no contributing immunosuppressant medications, relevant past medical history, prior or current malignancy, infective symptoms, or sexual history. Immunological testing revealed negative HIV serology during admission and 6 weeks post-admission and normal total levels of immunoglobulins A, M, and G, CD3+/4+/8+ T cells, CD19+ B cells, and CD3- natural killer cells. An infective screen was negative for serum HSV1/2 IgM, CMV IgM, and CMV viral load <30 IU/mL, Epstein-Barr virus IgM, and an infective screen of sexually transmitted infections was negative for Chlamydia trachomatis, Neisseria gonorrhoeae, and Syphilis. The patient did not have chronic viraemia or systemic infection with CMV or HSV. Additionally, in consultation with inpatient immunology specialists, the patient was deemed to have no immunodeficiency.
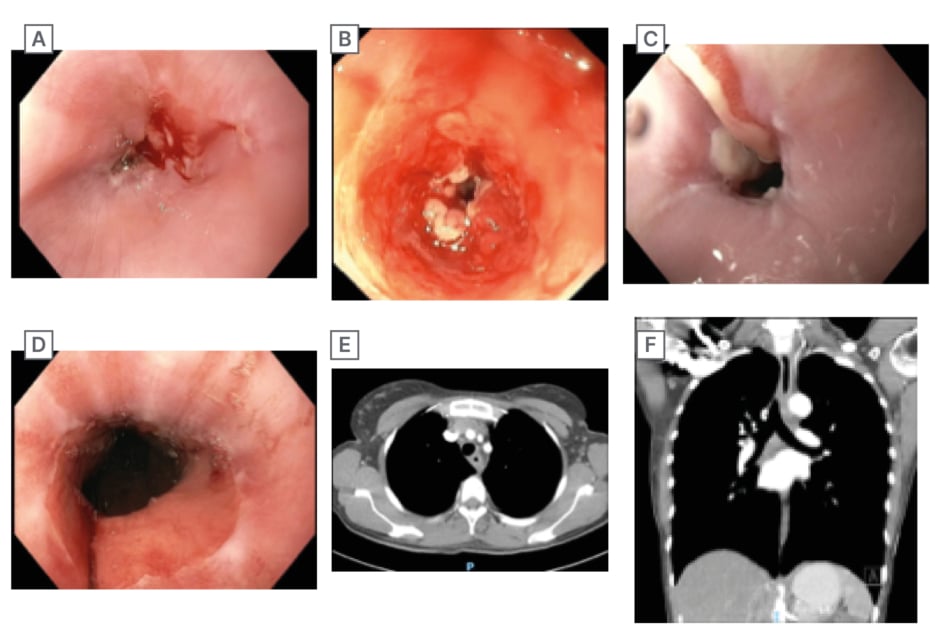
Figure 1: Gastroscopy and CT images.
A) Gastroscopy image showing the esophageal stricture during inpatient admission. B) Inpatient gastroscopy after attempt to traverse the stricture with a slim gastroscope. C) Prior to 5ᵗʰ oesophageal dilatation. D) Post 5ᵗʰ oesophageal dilatation showing marked improvement in luminal diameter. E) CT axial view of oesophageal stricture at 23 cm from the incisors showing concentric wall thickening. F) CT coronal view of oesophagal stricture.
Light microscopy showed stratified squamous mucosa with underlying granulation tissue and dense mixed inflammatory infiltrate, as shown in Figure 2. Immunohistochemistry demonstrated focal positive nuclear staining for HSV-1 and HSV-2 within the epithelium and positive CMV staining in the enlarged endothelial cells. Special stains were negative for other organisms. There was no dysplasia and no evidence of malignancy. The patient was commenced on valganciclovir, a high-dose proton pump inhibitor, and received optimised nutrition in conjunction with an inpatient dietician. Initially, nasogastric feeding was attempted but subsequently changed to total parenteral nutrition via a central line due to the patient not tolerating the nasogastric tube. The central line was removed following 3 kg of inpatient weight gain, and she was discharged with oral supplements. She also received an iron infusion of 1 g ferric carboxymaltose on discharge. Following discharge, the patient received weekly oesophagal dilatations via controlled radial expansion balloon in sequential increments of size for 5 weeks, also shown in Figure 1, with symptoms of dysphagia improving drastically and a further 4 kg weight gain over this time period. The patient was treated for a total of 6 weeks of oral valganciclovir and planned for ongoing outpatient follow-up.
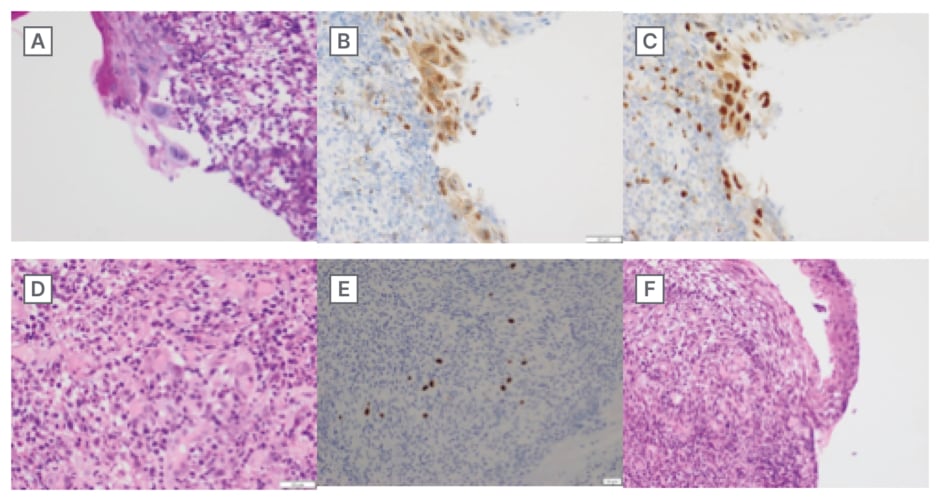
Figure 2: Histology images of oesophageal biopsy.
A) PAS stain showing nuclear features of HSV infection: enlarged multinucleated cells with chromatin marginalisation and nuclear molding. B) HSV-1 IHC stain showing infection with HSV1. C) HSV-2 IHC stain showing infection with HSV-2. D) H&E stain showing inflamed stroma with nuclear features of CMV infection: enlarged cells with basophilic nuclei and pale perinuclear regions. E) CMV IHC stain showing infection with CMV. F) H&E stain on lower magnification showing significant inflammatory infiltrate into the stroma of the biopsied oesophagus.
CMV: cytomegalovirus; H&E stain: haematoxylin and eosin stain; HSV: herpes simplex virus; IHC: Immunohistochemistry.
DISCUSSION
Infectious oesophagitis is the leading cause of oesophagitis worldwide.1 CMV is a common virus affecting the general population, with 50% of people being exposed by young adulthood and 85% by the age of 40.2 In immunocompetent individuals it can present with flu-like symptoms such as fever, fatigue, and muscle aches; however, in immunosuppressed patients, it can cause more severe symptoms and affect multiple organs, including the eyes, lungs, liver, and gastrointestinal tract.3 HSV is also a common virus in the population, with HSV-1 being more common than HSV-2, 67% versus 13% in individuals under 50, respectively.4 In immunocompetent individuals, infection with HSV presents with vesicular lesions generally on mucosal surfaces in contact with the external environment such as the mouth or genitals. The presentation of HSV in immunocompromised patients is known to affect other organs as well such as the liver, brain, and lungs causing more severe symptoms.5
CMV and HSV are both known to affect the oesophagus and are routinely screened for.6 Cases of oesophageal strictures have been noted in CMV oesophagitis but only in immunosuppressed adults and children, as seen in Table 1.7-13 Additionally, concurrent oesophagitis with CMV and HSV is rare and is documented almost entirely in case studies of HIV, transplant, or other medication-related immunosuppression.14 An oesophageal stricture refers to the formation of granulation tissue and a narrowing of the lumen which leads to dysphagia and is associated with chronic conditions such as gastroesophageal reflux disease, oesophageal cancer, and eosinophilic oesophagitis.15 Oesophageal strictures as a result of CMV and HSV infection are extremely rare and only documented in a single case series of patients with HIV.8
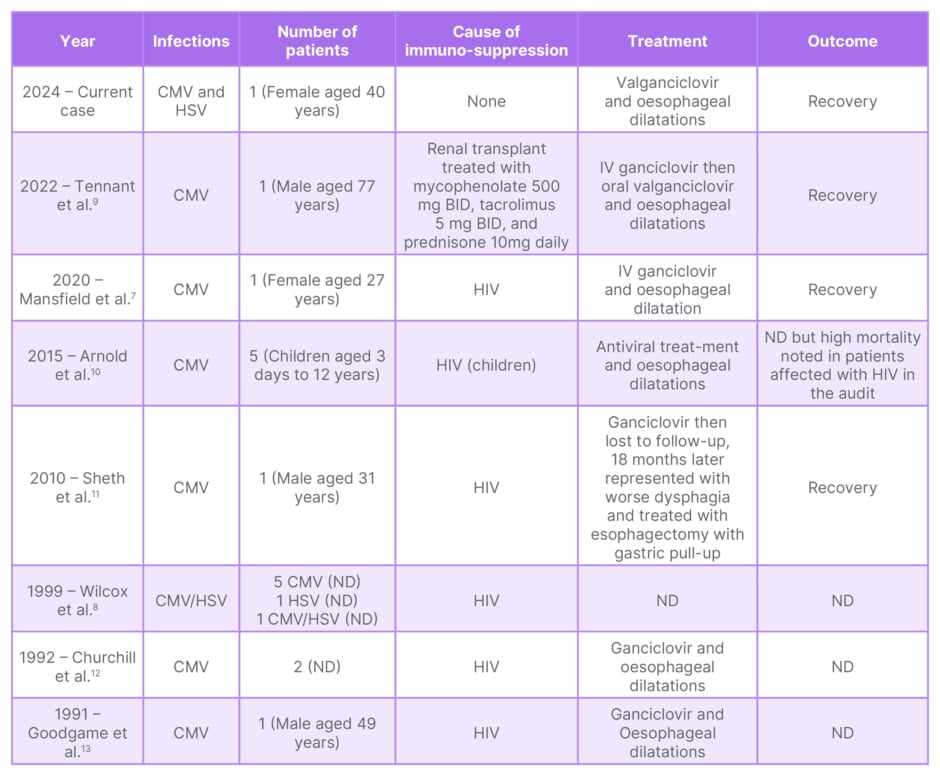
Table 1: Patients reported to have CMV-related strictures in the literature.
BID: twice a day; CMV: cytomegalovirus; HSV: herpes simplex virus; IV: intravenous; ND: not disclosed.
The authors present a case of a circumferential oesophageal stricture with associated concurrent CMV/HSV infection in a young immunocompetent woman. This is a novel presentation of viruses commonly associated with immunosuppression. Interestingly, ulceration of the oesophagus, an acute macroscopic feature of both CMV and HSV oesophagitis, was not seen on endoscopy. Acute infection was also excluded by serological testing of CMV and HSV. Histological testing, however, demonstrated clear CMV and HSV infection. As a result, the oesophageal stricture in this case is likely the result of chronic infection and subsequent fibrous healing, which has been postulated in previous case reports of CMV-related oesophageal strictures.16 However, it is unclear what the contributing factors were to CMV and HSV reactivation in this case, in the absence of clear causes of systemic immunosuppression. A control biopsy of the stricture following anti-viral treatment was not performed as the patient discharged her care to another health service internationally; however, this would have been interesting to ascertain the persistence of CMV or HSV in the oesophagus. Instead, treatment was considered successful with a lack of stricture progression and increased patency on serial dilatations with concurrent anti-viral treatment.
Local oesophageal microbiome changes have been identified in disease states of the oesophagus.17,18 A review of infectious esophagitis in children raises the possibility of local microbiome changes or dysbiosis in the oesophagus as a contributor towards eosinophilic oesophagitis, a chronic inflammatory process.19 As the presentation in this case involves a chronic inflammatory process, it is possible that dysbiosis may contribute. Influences on gastrointestinal microbiota include proton pump inhibitors, antibiotics, and diet, yet the impact of these on the oesophageal microbiome is incompletely understood.20 Additionally, the role of dysbiosis in augmenting the immune response as it pertains to CMV and HSV infection is not well understood but warrants further investigation.
CONCLUSION
Infectious oesophagitis is associated with significant morbidity in immunosuppressed populations. Concurrent CMV and HSV infection of the oesophagus is very rare in immunocompetent patients, which prompts the authors’ description of this case report. Oesophageal strictures are also associated with significant morbidity when symptomatic with dysphagia, weight loss, and malnutrition. Both infectious oesophagitis and oesophageal strictures warrant significant investigation to identify immunosuppression and malignancy. Further research on the role of dysbiosis in oesophageal pathology may provide insight into the pathophysiology of these diseases in the future.







