Abstract
Cefixime is a well-tolerated third-generation cephalosporin with severe side effects that are infrequently encountered. Herein, the authors report a clinical case of a 79-year-old female diagnosed with cefixime-induced hepatitis. To the best of the authors’ knowledge, a single reported case was documented in the medical literature but has not been supported by liver biopsy. This case highlights the need to suspect drug-induced liver injury with cefixime use.
INTRODUCTION
Cephalosporins, a family of bactericidal antibiotics, have side effects like penicillin due to the similarity in their basic structure. Hypersensitivity and drug allergy are often reported but hepatotoxicity and drug-induced liver injury (DILI) due to these agents have rarely been encountered. A special exception is ceftriaxone, which belongs to the third generation and, when given parenterally, can cause biliary sludge with symptoms of cholestatic jaundice and even cholecystitis.¹
Cefixime is one of the widely used cephalosporins in the treatment of urinary tract and abdominal infections caused by Gram-negative bacteria. It belongs to the third generation and is usually a safe and well-tolerated drug. Like all β-lactam antibiotics, cefixime binds to specific penicillin-binding proteins located inside the bacterial cell wall but with higher stability in the presence of β-lactamase enzymes, causing the inhibition of bacterial cell wall synthesis. It is metabolised by the liver and approximately 50% of the absorbed dose is excreted, unchanged, in the urine in 24 hours. Its known side effects are disturbances in bowel habits, mainly diarrhoea; dyspepsia; headache; fatigue; dizziness; and myalgias.² To the best of the authors’ knowledge, only one previous case of cefixime-related hepatitis has been reported in medical literature but no liver biopsy was completed to support the causality.³ Herein, the authors document a case of cholestatic hepatitis caused by cefixime, after ruling out all other causes. The diagnosis was supported by liver biopsy and confirmed by a positive challenge test in the second admission. This case highlights the need to suspect DILI with cefixime use.
CASE REPORT
The patient was a 79-year-old female non-smoker with a history of Type 2 diabetes mellitus and recurrent urinary tract infections, who presented at the authors’ hospital with jaundice. Three days prior to presentation, the patient started experiencing dysuria associated with chills and was consequently started on cefixime 400 mg once daily. Two days later, she started to have non-radiating epigastric pain, along with nausea, multiple episodes of vomiting, pruritis, and progressive jaundice. She also reported clay-coloured stools and dark urine. She denied any alcohol intake. She had no recent history of travelling. Her only current medication was repaglinide 2 mg twice daily, without herbal products or any other drug intake.
On presentation, the patient was haemodynamically stable, afebrile, not in distress, and had an icteric sclera with right upper quadrant and epigastric tenderness on abdominal examination. Her blood test results were as follows: white blood cells: 5.2×109/L, with neutrophilic shift; haemoglobin: 11.9 g/dL; creatinine (Cr): 0.91 mg/dL; urea: 53.0 mg/dL; aspartate aminotransferase: 266.0 U/L (normal: <40.0 U/L); alanine aminotransferase (ALT): 205.0 U/L (normal <40.0 U/L), γ-glutamyl transpeptidase: 178.0 U/L (normal: <55.0 U/L); total bilirubin: 7.4 mg/dL; direct bilirubin: 6.8 mg/dL; alkaline phosphatase (ALP): 276.0 U/L (normal: <140.0 U/L); total protein: 6.8 g/dL; albumin (Alb): 3 g/dL; prothrombin: 1.1 sec.
One month prior to admission, the patient’s liver enzymes were at normal levels at a routine check-up. Furthermore, viral serologies for hepatitis A, B, C, cytomegalovirus, and Epstein–Barr virus were all negative, as well as markers for autoimmune hepatitis (e.g., γ-globulin, smooth muscle actin antibody, antinuclear antibodies, and antimitochondrial antibodies) and iron profile was normal. An abdominal ultrasound showed that the gallbladder was distended, no calculi, no wall thickening, no biliary ductal dilatation, a slightly enlarged liver with homogeneous echotexture, no focal solid or cystic lesions, and normal pancreas and kidneys.
During hospitalisation, there was a persistent increase in liver enzymes as shown in Table 1, so a liver biopsy was scheduled and completed on the eighth day of admission. A liver biopsy showed a preserved hepatic lobular architecture, with no evidence of portal tract fibrosis, fibrous septa, or portal to portal bridging fibrosis and cirrhosis. Within the portal tracts sampled was a lymphocytic inflammatory cell infiltrate and few eosinophils (Figure 1). A focal area of necrosis (Figure 2) was seen, but no bile duct inflammation or damage, lymphoid aggregates, and plasma cell infiltrate was seen. Canalicular and hepatocyte cholestasis was prominent, with no evidence of steatosis, iron, or copper overload, granulomas, viral inclusions, or ground glass cytoplasm; however, Mallory bodies were noted. Consequently, intravenous methylprednisolone 80 mg was started daily due to the persistent increase in cholestasis and international normalised ratio (INR). A very good response, with gradual decrease in cholestasis and INR, is shown in Table 1. The patient was discharged on Day 18, with gradual steroid tapering over a period of 2 months. After 2 months her liver enzymes were back to normal values.
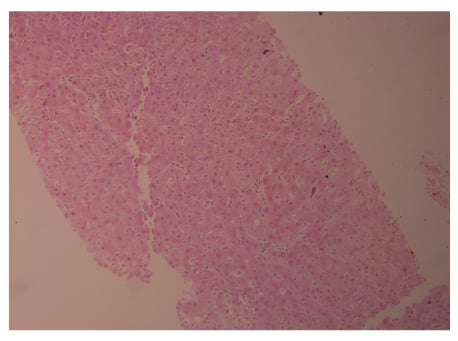
Figure 1: Acute and eosinophilic cell infiltrate.
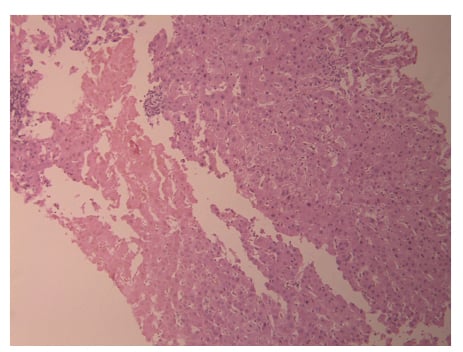
Figure 2: Portal tract inflammation, with focal area of necrosis, but with preserved architecture.
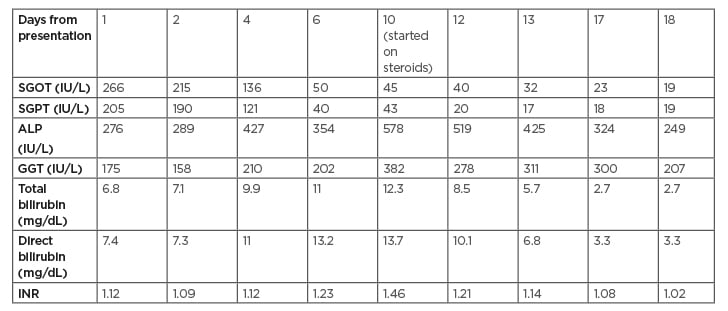
Table 1: Follow-up on liver enzyme tests, as documented during the admission.
ALP: alkaline phosphatase; GGT: γ-glutamyl transpeptidase; INR: international normalised ratio; SGOT: serum glutamic-oxaloacetic transaminase; SGPT: serum glutamate pyruvate transaminase.
Five months later, she presented again with jaundice, pruritis, dark urine, and clay-coloured stools, one day after taking cefixime for a urinary tract infection prescribed by another physician. Her laboratory test results were as follows: white blood cells: 7.2×109/L; haemoglobin: 11.5 g/dL, Cr: 0.8 mg/dL; urea: 43.0 mg/dL; aspartate aminotransferase: 184.0 U/L; ALT: 240.0 U/L; γ-glutamyl transpeptidase: 273.0 U/L; ALP: 488.0 U/L; total bilirubin: 8.9 mg/dL; direct bilirubin: 7.8 mg/dL; total protein: 6.8 g/dL; Alb: 3.5 g/dL; prothrombin: 1.1 sec. Cefixime was stopped and her liver enzymes showed a gradual amelioration without starting steroids, and the patient was discharged on Day 3 of admission. At a 1-month follow-up her liver enzymes were back to normal values. According to the Roussel Uclaf Causality Assessment Method (RUCAM) score, it was “highly probable” that cefixime was the cause of the liver injury in this case.⁴
DISCUSSION
The authors’ patient presented with jaundice and her laboratory results showed cholestatic hepatitis. She had no history of liver disease and there was no encephalopathy or an increase in INR >1.5 during her hospitalisation. Therefore, acute liver failure and acute-on-chronic liver failure were ruled out. All causes of hepatitis and perturbation of liver function tests, including viral serologies, autoimmune hepatitis, haemochromatosis, choledocholithiasis, and tumours, were ruled out.
DILI usually occurs 5–90 days following drug ingestion. In this case, the injury occurred 3 days after the intake of cefixime in the first presentation and 1 day after in the second presentation.
It is noteworthy that DILI is traditionally classified as intrinsic (or direct) versus idiosyncratic. Direct DILI is typically dose-related. Its onset is within a short time span (hours to days), and it occurs in a large number of individuals exposed to the drug (predictable). Whereas idiosyncratic DILI is not usually dose-related, it requires a dose threshold of 50–100 mg/day and exhibits a variable latency to onset, ranging from days to weeks. It occurs in only a small proportion of exposed individuals (unpredictable).⁵
The mechanism of DILI related to cefixime needs to be elucidated but it is, most probably, idiosyncratic. The diagnosis is usually difficult due to the lack of specific symptoms, signs, and tests and is, in part, a diagnosis of exclusion. The clinical spectrum of drug-induced hepatotoxicity is widely variable, ranging from asymptomatic elevation of liver enzymes to fulminant hepatic failure. Thus, comprehensive clinical assessment is a must to establish the diagnosis. Typically, history indicates a suspect drug, with reasonable temporal association to the illness. A pattern of liver injury, characterising the effect of the drug, is also helpful in diagnosis.⁶
According to the Councils for International Organizations of Medical Sciences (CIOMS), DILI may present as hepatocellular, cholestatic, and mixed.⁷,⁸ Hepatocellular injury is characterised by an elevation of liver enzymes by ALT ≥3 times the upper limit of normal (ULN), and ALT/alkaline phosphatase (R ratio) ≥5 times the ULN. Cholestatic injury consists of an ALP elevation of ≥2 times the ULN and an ALT/ALP ratio of ≤2 times the ULN. Mixed type is established when ALT is ≥3 times the ULN, ALP is ≥2 times the ULN, and the ALT/ALP ratio is <5 but >2 times ULN.⁹ During the first days, the type of injury in the authors’ case seemed to be classified as a mixed pattern, and became a cholestatic pattern in the following days. However, in the other case reported in the literature it was classified as hepatocellular pattern on diagnosis.³
Drug-induced hepatitis is a diagnosis of exclusion but should be suspected¹⁰ when a new drug has been started in the past 3 months; there is mixed-type liver injury; the presence of a rash or eosinophilia; cholestasis, with no biliary obstruction on imaging; hepatitis without hypergammaglobulinemia or autoantibodies. The liver biopsy helps in identifying alternative aetiologies. In addition to the fact that it raises or lowers the likelihood of a particular drug injury based on reported patterns,¹¹ especially when there is persistent deterioration of liver enzymes, as in the authors’ patient.
In most cases, the histologic findings of DILI are as follows: demarcated perivenular necrosis; canalicular cholestasis with minimal hepatitis; poorly developed portal inflammatory reaction; neutrophilic infiltration; eosinophilic infiltration; and granulomatous with epithelioid cells.¹² The authors’ patient’s liver biopsy showed that cholestasis excluded all other aetiologies of liver injury and supported the diagnosis, but the positive challenge test that was completed by mistake in the second presentation confirmed it. The only new drug started was cefixime, and because the symptoms started 2 days after starting it in the first presentation and 1 day after starting it in the second presentation, cefixime was responsible of this liver injury and cholestasis.
Management of DILI consists of rapid discontinuation of the offending suspected drug.¹³,¹⁴ In fact, no beneficial therapies were reported except the use of N-acetyl cysteine for acetaminophen hepatotoxicity,¹⁵,¹⁶ and the use of ursodeoxycholic acid and steroids in cholestatic DILI,¹⁷ with circumstantial success;¹⁸ however, a targeted treatment for idiosyncratic injury remains to be found.¹⁹
Given the anti-inflammatory effects of corticosteroids, they have been widely used for the treatment of DILI. Although multiple studies and case reports have shown a beneficial effect of corticosteroids in DILI,²⁰ a recent observational study of 90 patients refuted this hypothesis in patients with severe DILI.²¹ Therefore, the efficacy of corticosteroids in DILI remains controversial and a prospective controlled trial will be needed to confirm or refute their beneficial effect in DILI. Whereas, in the authors’ patient, the persistent increase in cholestasis lead to steroids treatment with a very good outcome in the first presentation; however, in the second presentation, the cholestasis resolved by stopping the offending drug without steroid treatment.
CONCLUSION
The liver is responsible for metabolising a majority of medications, which make this organ a prime target for drug-induced damage, which can manifest in abnormal liver tests without any symptoms suggestive of liver disease. The authors’ case is the second case in the medical literature reporting cefixime-induced hepatitis and the first one supported by liver biopsy and confirmed by a positive challenge test. Treatment with steroids was of benefit in the first presentation, whereas stopping the offending drug led to the normalisation of liver enzymes in the second presentation. This case of hepatotoxicity highlights the importance of considering cefixime as potential cause of liver injury in the future.








