Abstract
Cancer cachexia is highly prevalent among patients with the advanced stage of cancers and leads to a higher risk of mortality. Delayed management of cachexia results in suboptimal treatment outcomes and irreversible progression to refractory cachexia. The purpose of this review is to provide the pathophysiology of cancer cachexia, emerging diagnostic criteria with potential biomarkers, prevention strategies, and novel treatment approaches. Cachexia is characterised by the presence of an inflammatory process in conjunction with muscle mass and unintentional body weight loss. Various biomarkers such as leptin, ghrelin, TNFα, essential amino acids, total amino acids, and C-reactive protein are indicative of cachexia. Increased circulating levels of β-dystroglycan, myosin heavy-chain, and dystrophin are indicators of shortened survival time as skeletal muscle tissues break down. Despite muscle wasting being a hallmark of cachexia, recommended cachexia management is limited to nutritional counselling and administration of an appetite stimulant and corticosteroids for a short period, which often fail to reverse cancer cachexia. It is critical to monitor weight loss using the cachexia grading system for early detection, to halt progression to refractory cachexia and improve the survival of patients with cancer cachexia.
INTRODUCTION
Cancer cachexia is highly prevalent among patients with the advanced stages of cancers, affecting an estimated 12 million people worldwide and being causative in up to 2 million deaths annually as of 2016.1 A distinguishing feature of cachexia is the loss of musculoskeletal lean body mass, with or without fat mass loss, in conjunction with weight loss of >5% over the course of 6 months.2 These metabolic derangements delineate it from age-related sarcopenia or malnutrition, which can be reversed with proper nutritional supplementation or exercise.3
Cancer-associated cachexia is often linked to increased morbidity and mortality given its underdiagnosis and delayed treatment.4,5 Delayed diagnosis of cachexia decreases quality of life, and may delay optimal patient care if systemic inflammation and gastrointestinal (GI) symptoms hinder the administration of necessary chemotherapy.6 Approximately 80% of patients with advanced cancers experience cachexia, at which point intervention measures are often too late to reverse the condition and the progressive nature of the malignancy is accelerated by the complex cachectic metabolic derangement. Patients with advanced GI cancers may present with a higher incidence of cachexia given the aggressive nature of chemotherapy, the nutritional deficiency caused by the malignancy, and the relative proximity of localised inflammation and systemic responses.7 This review addresses the pathophysiology of cancer cachexia, emerging diagnostic criteria and potential biomarkers, prevention strategies, and current as well as novel treatment approaches that either slow, halt, or reverse the progression of cachexia in patients with GI cancer.
PATHOPHYSIOLOGY OF CANCER CACHEXIA
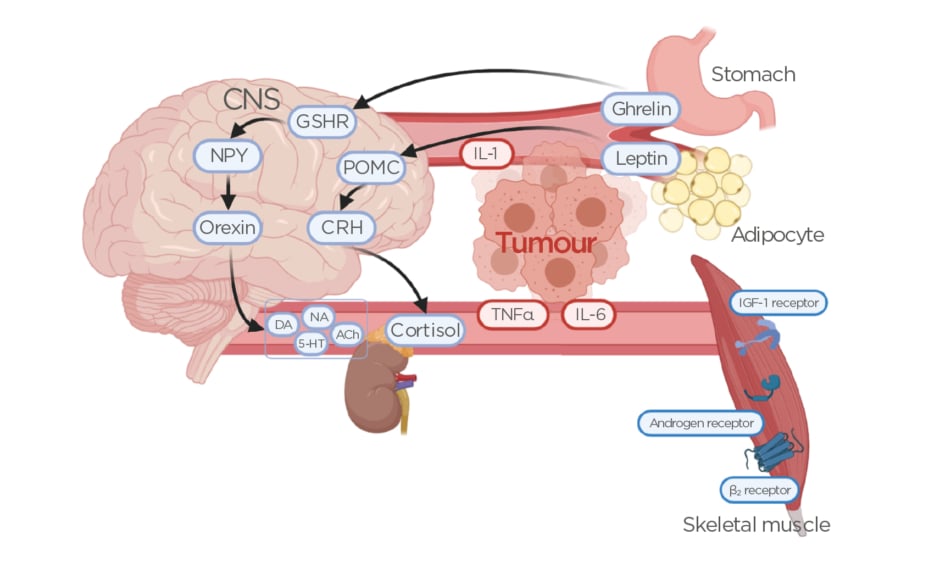
The multifactorial nature of cancer development itself is highly complex, so it comes as no surprise that the pathophysiology of cachexia within this specific setting remains poorly understood. However, cachexia can be clearly differentiated from both malnutrition and sarcopenia by the presence of an inflammatory process in conjunction with muscle mass and body weight loss.3 The systemic metabolic derangement caused by the malignancy is a result of hypercatabolism, hypermetabolism, systemic inflammation, and an imbalance in protein synthesis regulation.8,9 Regulation of caloric intake is mediated through a variety of hormones, among them the adipocyte-generated cytokine-associated hormone leptin, the orexigenic peptide ghrelin present primarily in the GI tract, and the neuropeptide α-melanocyte-stimulating hormone.10-12 Under physiological conditions, upon food intake leptin is released into the blood circulation to stimulate the production of proopiomelanocortin, leading to the release of cortisol and ultimately suppression of further food consumption (Figure 1).13 Its opposing hormone, ghrelin, stimulates appetite by increasing the release of neuropeptide Y and orexin in the central nervous system, leading to increased gastric acid secretion (Figure 1).13,14 A higher amount of leptin is expressed in patients with GI cancers, while a lower amount of ghrelin is present in this population, contributing to a deranged metabolism and lower caloric intake.15 The imbalance between leptin and ghrelin is primarily attributed to systemic inflammation, an early hallmark of cancer and a necessary contributor to the development of cachexia. In fact, weight loss of <5% over 6 months but increasing inflammatory markers may indicate a precachectic state that warrants intervention to prevent progression.16,17 It has also been observed that patients with cachexia develop resistance to ghrelin even if the hormone is being supplemented to stimulate appetite.18
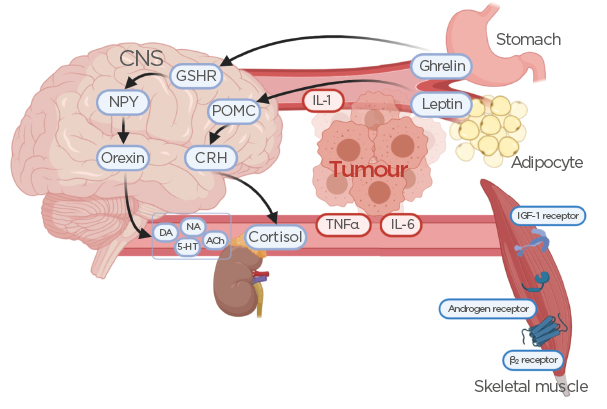
Figure 1: Effects of ghrelin and leptin secretion on the release of hormones and neurotransmitters from the central nervous system.
Release of inflammatory mediators from the tumour alters ghrelin and leptin homeostasis, leading to reduced skeletal muscle tissue. Also shown are potential targets for pharmacotherapeutic intervention, such as GSHR, IGF-1 receptor agonists, androgen receptor agonists, and adrenergic β2 receptor antagonists.
ACh: acetylcholine; CNS: central nervous system; CRH: corticotropin-releasing hormone; DA: dopamine; GSHR: ghrelin receptor agonists; IGF-1: insulin-like growth factor-1; NA: noradrenaline; NPY: neuropeptide Y; POMC: proopiomelanocortin; 5-HT: serotonin.
A nonspecific marker of systemic inflammation is C-reactive protein (CRP), which increases in the early stages of malignancy. More specific to GI cancers are elevated plasma levels of TNFα, IL-1β, and IL-6, as well as a reduction in serum albumin and adiponectin levels.15 While TNFα is not a specific marker of cancer cachexia, its increased blood levels in conjunction with rising IL-6 levels correlate with the progression from malnutrition to cachexia (Figure 1).15 Interestingly, expression levels of IL-1β were found to be elevated in adipose and tumour tissues of GI cancer patients with cachexia versus those without cachexia, indicating more pronounced crosstalk between the tumour and surrounding tissues as a contributing factor in the development of cachexia.19 Along with higher IL-1β expression in patients with cachexia comes an increase in fibrosis and macrophage infiltration in subcutaneous adipose tissue compared to weight-stable patients with cancer, suggesting both an inflammatory and morphological differentiation in GI cancer cachexia.20
Because of a shift in metabolic and catabolic activity, both lipid and protein, as well as muscle-related biomarkers, may indicate sarcopenia and malnutrition. Increased local and systemic inflammation due to the tumour cause muscle proteolysis, in conjunction with malabsorption of nutrients due to the localised presence of the malignancy. The ratio of essential amino acids to total amino acids and CRP in plasma was higher in patients with GI cancer who lost psoas muscle area compared to those who maintained or gained psoas muscle.21 This association between inflammation, increased proteolysis, and loss of muscle mass indicates that patients with advanced GI cancer have deranged metabolic/catabolic activity that cannot be compensated with nutritional supplementation alone. Other indicators of loss of muscle mass are increased plasma levels of β-dystroglycan, myosin heavy-chain, and dystrophin, which play a vital role in providing structural integrity to muscle tissues.22 Increased circulating levels are indicators of shortened survival time and refractory cachexia as skeletal muscle tissues are breaking down.
EMERGING DIAGNOSTIC CRITERIA AND BIOMARKERS
Patients with GI cancer remain at high risk of developing cancer cachexia and a majority are diagnosed too late for effective prevention or treatment to slow or reverse the progression of muscle and weight loss.
Delayed diagnosis leads to increased morbidity and mortality, lower quality of life, and suboptimal therapeutic outcomes.4,5 Early and frequent evaluation of patients with GI cancer is of critical value to detect weight loss as well as early changes in clinical and metabolic status. Such changes fall in the category of precachexia if inflammatory and/or nutritional markers are changing, and nutritional needs evolve either independently or based on chemotherapy or radiation treatment (Table 1).23 The most common diagnostic criteria are nutritional assessments, weight loss >5% over 6 months without starvation, >2% of weight loss if BMI <20 kg/m2, and/or demonstration of sarcopenia via skeletal muscle index measurement.2
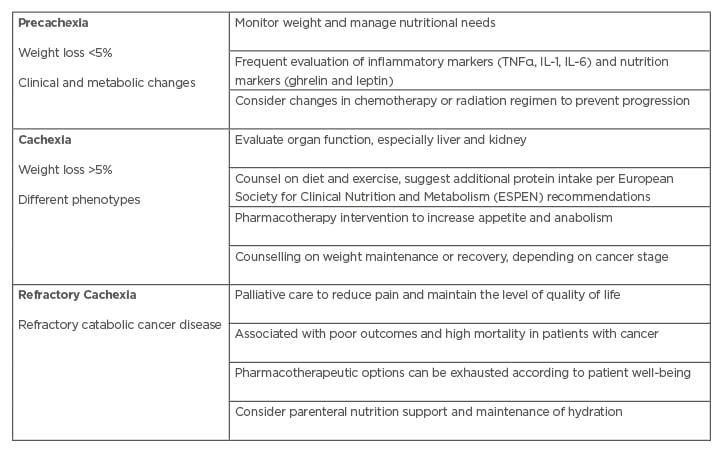
Table 1: Stages and characteristics of cachexia.
Adapted from Grundmann et al.23
Nutritional assessment tools are often used in conjunction with weight changes and quality of life observations in diagnosed patients. Among the established scales, the Nutrition Risk Screening-2002 (NRS-2002), Malnutrition Universal Screening Tool (MUST), and the Malnutrition Screening Tool (MST) are frequently used in clinical practice to establish nutritional and metabolic derangements.16 If a patient has been identified to be at risk for developing cachexia or is precachectic, a more in-depth evaluation of nutritional intake, physical activity, and body composition has to be considered, along with nutrition assessment tools, such as the Subjective Global Assessment (SGA) or Minimal Nutrition Assessment (MNA), to evaluate the degree of malnutrition and existing cachexia.16,24 The combined use of physical diagnostic criteria and nutritional screening or assessment tools has been given strong recommendations by the European Society for Clinical Nutrition and Metabolism (ESPEN) despite a “very low” level of available evidence.16,25 This has been further refined as a two-step model for risk screening and diagnosis assessment of malnutrition by the Global Leadership Initiative on Malnutrition (GLIM), first convened in 2016.26
The latest GLIM criteria as of 2019 include unintentional weight loss, low BMI, and reduced muscle mass as phenotypic criteria, and reduced food intake and inflammation or disease burden as aetiologic criteria, of which at least one phenotypic and aetiologic criterion need to be present to diagnose malnutrition.26
Patients with a weight-stable condition and BMI ≥25 kg/m2 demonstrated longer survival than the patients who lost weight.27 While cachexia is characterised by lean muscle loss, the association of BMI by % of weight loss can predict the prognosis of patients with cachexia, quality of life, and symptom burden. The cachexia grading system (ranged 0–4), based on % of weight loss and BMI, is beneficial for early detection to manage cancer cachexia.27,28
Thus, the ideal goal would be to prevent the development of cancer cachexia in the first place by recognising potential cachexia in patients with GI cancers. Once cachexia has progressed past a particular point, muscle degradation and loss of physical functioning are irreversible and impact the success of therapy and outcome. Patients with GI cancers are at higher risk of death if they had developed cachexia or refractory cachexia, lower grades in phase angle, decreased handgrip strength, and an increased CRP.29 Together with weight loss, these measures can be utilised to evaluate the progression of muscle strength loss and increased inflammation to provide potential intervention. The phase angle, a composite measure obtained by bioelectrical impedance analysis, can predict nutritional status and overall health status.30
Another early marker of cachexia in GI cancer is carnosine dipeptidase 1, which has been associated with weight loss, malnutrition, lipid breakdown, and low circulating albumin as well as insulin-like growth factor 1.31 The plasma levels of the enzyme, which plays a role in several disease states and is primarily expressed in the central nervous system and the liver, is significantly reduced in patients who develop cachexia compared to weight-stable patients with GI cancer. Carnosine is highly concentrated in muscle tissue, serving as a pH buffer to balance aerobic and anaerobic metabolism and catabolism activity.32 Because muscle integrity and degradation is a contributing factor to cachexia, elevated plasma levels of β-dystroglycan can serve as specific biomarkers for the diagnosis of GI cancer cachexia, while elevations in dystrophin and myosin heavy-chain may predict poor survival.22 Another potential predictor of muscle degradation in patients with GI cancer with cachexia are serum levels of carnitine, an essential compound needed in fatty acid energy metabolism in skeletal muscle cells.33 Carnitine serum levels were significantly lower in patients with GI cancer with cachexia compared to other patients with cachexia and healthy controls, potentially providing a specific marker for the severity of cachexia in patients with cancer.
Despite these emerging biomarkers for cachexia development and progression, none are routinely used in clinical practice or have been tested widely as screening tools.
Current clinical practice guidelines for cachexia diagnosis primarily rely on the overall patient status by evaluating subjective symptoms, taking a history, a clinical examination, body composition measures, general laboratory values, and activity monitoring (Table 2).16,34 While this approach can identify cachexia, it is often not specific or sensitive enough to monitor the development of progression in a timely manner for providing appropriate intervention. Clinicians may, therefore, consider additional laboratory measures as discussed above to guide important pharmacologic and nonpharmacologic treatment decisions to prevent or halt the progression of cancer cachexia before it advances to the mostly treatment-resistant refractory cachexia stage (Table 1).
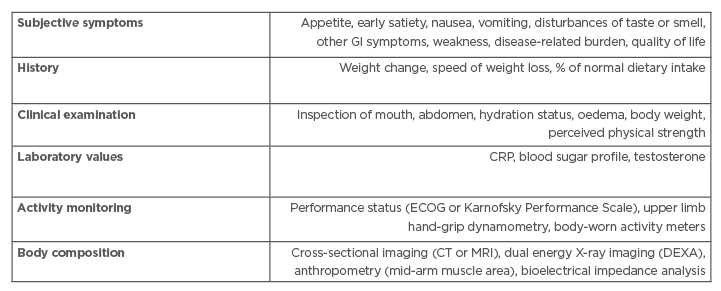
Table 2: Current guidelines to diagnose cancer-associated cachexia.
CRP: C-reactive protein; DEXA: dual-energy X-ray absorptiometry; ECOG: Eastern Cooperative Oncology Group; GI: gastrointestinal. Adapted from Radbruch et al.34
PREVENTION STRATEGIES FOR GASTROINTESTINAL CANCER CACHEXIA
Cancer cachexia contributes to an increased risk of premature death in patients with GI cancer; hence, preventing its development remains a primary challenge and opportunity to improve quality of life and patient outcomes. Given that sudden and unexpected weight loss is both a hallmark indicator for tumour growth and anorexia-cachexia, it may serve as a nonspecific but leading sign for clinicians to investigate further. Any patient diagnosed with cancer is at risk of developing cachexia and therefore should be frequently monitored for weight loss, changes in appetite and caloric intake, decrease in muscle strength, and increased inflammation.
Given the profound loss of lean muscle mass and metabolic derangement, nutritional intervention serves as an initial and ongoing therapeutic intervention to prevent or potentially halt or reverse the progression of cancer cachexia. Specifically, protein intake should be increased to at least 1 g/kg/day, ideally to 1.5 g/kg/day, in combination with regular physical activity or exercise.16 Regular physical activity or exercise in conjunction with adequate nutrition is essential to maintain muscle strength, physical functioning, and metabolic activity.35
Rising serum levels of CRP, TNFα, and IL-6, in conjunction with >5% weight loss over 6 months and decreased muscle strength, is a strong indicator for a cachexia diagnosis.36 Hence, a prevention strategy that is commonly employed in patients with cancer has been physical exercise to maintain muscle strength and nutritional support for caloric intake.37,38 Physical exercise has been studied in several clinical trials for the prevention and treatment of cachexia in patients with cancer, and evaluated in a systematic Cochrane review.39 Despite agreement among clinicians and researchers that exercise does benefit patients with precachexia and cachexia, heterogeneity in study design and neglect to include cachexia staging and assessment prevent consistent evaluation of the safety and efficacy of exercise in cachexia. Hence, its benefit remains undetermined and clinicians are left to consider its recommendation on an individual patient basis. Similar to physical exercise, nutritional support is a commonly employed and clinically utilised adjunct therapy to prevent and treat anorexia, malnutrition, and cachexia.
However, enteral nutrition support with omega-3 fatty acids, arginine, glutamine, and polyribonucleotides has not shown consistent improvements or increased survival in patients with GI cancer, and may only benefit patients with good functional status and an overall better prognosis.40,41 Systemic inflammation remains a major contributing factor in the development of cancer cachexia and also serves as a biomarker for its diagnosis as previously discussed. Both the tumour and the immune response contribute to the development of a precachectic state that leads to a metabolic instability, hastening weight and muscle loss.42
Reducing or suppressing systemic inflammation can potentially reduce both the progression of the malignancy as well as the development of a cachectic state. The use of anticytokine drugs, such as thalidomide, that target a range of proinflammatory cytokines (TNFα, IL-6, IL-1β, etc.) has shown mixed results in treating or preventing cachexia in clinical trials to date, primarily due to a heterogeneous patient population and testing in late disease states.43 There is a potential correlation between the use of anti-inflammatory drugs, in particular the long-term use of aspirin, and lowering the risk of colorectal cancer development, as has been shown in several longitudinal studies.44,45
CURRENT AND NOVEL APPROACHES TO CANCER CACHEXIA TREATMENT
The two primary goals in the prevention and treatment of cancer cachexia are to increase or maintain appetite and to prevent a loss of muscle mass. Although weight and appetite loss are associated, they are not always an indication of cachexia since chemotherapy and radiation therapy themselves can impact appetite and weight changes through systemic and local inflammation themselves.46 Because the imbalance in catabolism and anabolism is caused by systemic inflammation, the most common first-line pharmacotherapies remain glucocorticoids and progesterone derivatives that aim to stimulate appetite and maintain or increase weight via anabolism.47,48 However, both drug classes have limited long-term benefits and do not improve physical functioning. Megestrol acetate remains the primary agent used to prevent and treat all stages of cancer cachexia and clinical studies indicate weight stabilisation or weight gain with its short-term use.49 Combination therapy with nonsteroidal anti-inflammatory agents to suppress inflammation did not show a benefit over treatment with megestrol acetate alone, and thus remains limited to use in clinical trials.50,51 Other agents that are occasionally used with mixed or equivocal success are cannabinoids, anticytokine and anabolic agents, and β-blockers (Figure 1).23 Each class of agents has limitations and none of the experimental off-label uses has shown consistent benefits in halting or reversing cachexia in patients with GI cancer specifically or patients with advanced cancer in general. Such is the case with the combined use of agents that stimulate protein synthesis, such as short-term use of glucocorticoids or omega-3 fatty acids,52 and agents that prevent catabolism, specifically thalidomide, which downregulates the ubiquitin-proteasome proteolysis pathway involved in protein degradation.48 Thalidomide remains controversial due to its known genotoxic and other adverse effects and has not been approved for the prevention or treatment of cancer cachexia. Its benefit in this population is also questionable given limited evidence from clinical studies.53 Newer drugs that are being investigated target specific signalling pathways involved with food intake. Among them is the ghrelin receptor agonist anamorelin that has shown some benefits in patients with non-small cell lung carcinoma (ROMANA1 and ROMANA2 studies, including a total of 979 patients)54,55 and one multicentre study in 50 patients with GI cancer.56 Anamorelin does modestly improve body weight and lean body mass over the course of 12 weeks in patients with cancer cachexia compared to those receiving placebo; however, the European Medicines Agency (EMA) did not grant market approval for this indication in non-small cell lung cancer. Aside from increased strength exercises, physical functioning remains unaddressed in clinical trials to date.
Pharmacological approaches have limitations due to side effects and added burden to patients with cachexia, who are taking multiple medications and experiencing treatment-associated side effects. Several nonpharmacological approaches are utilised to improve the quality of life and mitigate the limitations of current pharmacotherapy to overcome the adverse effects of cancer chemotherapy. Such approaches involve targeted acupuncture to reduce specific GI and cachexia symptoms,57 nutritional counselling, psychosocial interventions, and dietary supplements.58 Recent guidelines recommended using enteral tube feeding and parenteral nutrition only with caution, and not treating patients with these approaches consistently.59
CONCLUSION
Cancer-associated cachexia has received increased attention for the last two decades. The definition of cachexia has been generally agreed upon in the cachexia research community; however, diagnostic measures using the biological markers are still mainly under investigation. Recently, evaluation of the skeletal muscle index and psoas muscle index is often used to assess the loss of muscle mass for measuring cachexia. Despite various pharmacological agents having undergone clinical trials and some shown promising results, currently no medications are available to treat cachexia. Most recent guidelines for cancer cachexia management recommend dietary counselling, megestrol acetate as an appetite stimulant, or short-term use of dexamethasone.58 These medications may be helpful to stimulate appetite but should not be taken for a long time due to various side effects. Also, these medications or nutritional counselling may not slow down lean muscle loss or treat cachexia. If possible, nonpharmacological approaches that would not be burdensome for patients may be a promising solution for patients with cachexia, who are affected by fatigue, decreased energy levels, nausea, and decreased appetite.