Summary
Excess production of TNF-α leads to chronic inflammation and tissue damage in immune-mediated inflammatory diseases (IMID) such as Crohn’s Disease (CD), rheumatoid arthritis, and psoriasis. The introduction of anti-TNF agents revolutionised therapy for patients with IMIDs, and two anti-TNFs are currently approved for use in CD in Europe: infliximab and adalimumab. The chronic transmural inflammation associated with CD commonly leads to intestinal complications such as intra-abdominal abscesses, which present a challenge to a multidisciplinary medical team. While surgical management may ultimately be required in some patients, it is associated with a risk of morbidity and loss of function, particularly in a patient who requires immunosuppression to address their underlying CD. This mini review summarises the current evidence surrounding the use of anti-TNFs in CD complicated by intra-abdominal abscess, including current treatment guidelines, the use of anti-TNFs in combination with conservative (non-surgical) management, and the impact of anti-TNFs on post-operative complications and abscess recurrence.INTRODUCTION
The introduction of anti-TNF agents into the armoury of treatments for IMIDs revolutionised therapy for patients with diseases such as rheumatoid arthritis, axial spondyloarthritis, psoriasis, and inflammatory bowel disease (IBD).1,2 There are five novel anti-TNFs approved for use in IMIDs: adalimumab, infliximab, golimumab, etanercept, and certolizumab pegol,3-7 and multiple biosimilars have now also reached the market.8,9
Among these anti-TNFs, infliximab and adalimumab are approved for CD in the European Union (EU) and USA.3,4,10,11 Additionally, certolizumab pegol is approved in the USA for this indication, although marketing authorisation was refused by the Committee for Medicinal Products for Human Use (CHMP) in the EU, due to concerns regarding safety and efficacy in patients with CD.12
The chronic, transmural inflammation that occurs in CD commonly leads to intestinal complications such as strictures, fistulas, and abscesses.13-16 Intra-abdominal abscesses (as opposed to perianal abscesses) complicate the clinical course of CD in 10–30% of patients.17-19
The management of intra-abdominal abscesses presents a challenge to multidisciplinary medical teams.19,20 It is unclear whether a conservative approach of percutaneous drainage is sufficient, or whether it may serve as a bridge to surgical intervention, with the associated risks of morbidity and loss of function.14,20,21
Evidence from a long-term follow-up of patients in the MICA study was recently presented at the 2022 American Gastroenterological Association (AGA) Summit, suggesting that the use of adalimumab following percutaneous drainage may be an effective, well-tolerated approach to managing intra-abdominal abscess in select patients with CD.22 In view of these findings, this mini review summarises the current evidence surrounding the use of anti-TNFs in these complications.
INTRA-ABDOMINAL ABSCESS IN CROHN’S DISEASE
There are three mechanisms by which intra-abdominal abscesses can form in CD: first, the direct, transmural passage of bacteria from the diseased bowel into surrounding tissues; second, the passage of bacteria through the blood stream to remote tissues; and third, peritoneal contamination resulting from bowel surgery.23 Spontaneous abscess formation and post-operative abscess formation occur at similar rates in CD.23 Complex interactions between bacteria and the host immune response result in an accumulation of neutrophils and encasement of the infection in a fibrin sheath, forming an abscess.23
The management of CD with an intra-abdominal abscess is challenging because active and severe immune-mediated disease should ideally be treated with immunosuppressive medication, yet immunosuppression can be dangerous in the presence of a severe abdominal infection and when performing abdominal surgery.13,23
To avoid invasive bowel surgery, which carries the risk of post-operative sepsis, treatment with antibiotics with or without percutaneous drainage of the abscess is recommended as the first-line treatment for CD complicated by intra-abdominal abscess.13,24 Once the infection is controlled, CD medication can be restarted to prevent the recurrence of abscess formation.13
CURRENT CLINICAL GUIDELINES
Both the European Crohn’s and Colitis Organisation (ECCO) and the British Society of Gastroenterology (BSG) recommend that intra-abdominal abscesses complicating active CD should be managed with antibiotic therapy and either percutaneous (imaging-guided placement of a needle or catheter through the skin) or surgical drainage, followed by delayed bowel resection if necessary.25,26 If successful in treating sepsis, management with antibiotics and percutaneous drainage is likely to result in lower morbidity and stoma rates compared with surgical resection.26
BSG guidelines also recommend joint medical and surgical discussions following treatment of an abdominal abscess.26 Surgical resection is not always required; however, it is considered mandatory in the context of free peritonitis, and is highly likely to be performed in the setting of localised abscess formation.26 If surgery is required, it should follow patient optimisation in terms of sepsis control and treatment of nutritional deficiencies.26
The recent update of the French National Consensus (FNC) for IBD management also recommends percutaneous drainage and antibiotics at first line, but only if the intra-abdominal abscess is more than 3 cm in diameter.27 The FNC advises that antibiotic treatment should be maintained until the abscess is revaluated by magnetic resonance enterography 3–4 weeks after drainage.27 Regardless of the therapeutic strategy employed, FNC experts agree that enteral nutrition and preventative low molecular weight heparin (in high-risk cases) should be used.27
In terms of immunosuppressive therapy for the underlying CD, the FNC recommends anti-TNF combination therapy following abscess resolution, unless the abscess occurred while the patient was taking anti-TNFs. In the latter scenario, surgical resection was the preferred approach in cases with <50 cm of ileal disease, while second-line drugs were recommended in cases affecting >50 cm of the ileum (Figure 1).27
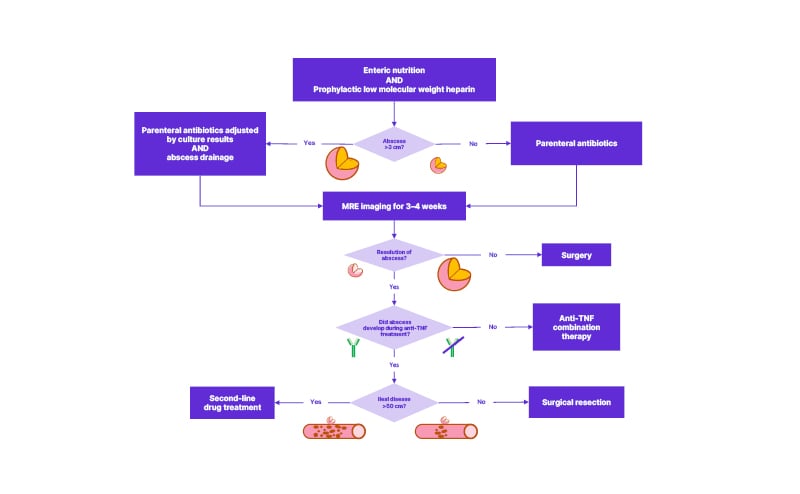
Figure 1: Treatment algorithm for complicated Crohn’s disease with an intra-abdominal abscess.
Adapted from Amiot et al.27
MRE: magnetic resonance enterography.
Guidelines from the American College of Gastroenterology (ACG) agree with those from European associations in terms of first-line therapy with antibiotics and percutaneous/surgical drainage.14 The USA guidelines also indicate that following drainage, most patients benefit from surgical resection, while some may benefit from medical therapy, although comparative studies have not been performed.14
Corticosteroids are relatively contraindicated in patients with intra-abdominal abscess-complicated CD,14 and the BSG suggests that exclusive enteral nutrition may be an effective way to control intestinal inflammation without use of corticosteroids.26
CLINICAL EVIDENCE
Prior to the widespread use of anti-TNF therapy for CD, several studies assessed the efficacy of conservative management (antibiotics and percutaneous drainage) of intra-abdominal abscesses. A meta-analysis performed using studies published between 1975 and 2015 found that conservative management alone was effective in approximately 30% of patients with CD-related intra-abdominal abscess.28 In another study, the median length of hospitalisation was reported to be significantly lower in patients receiving conservative therapy compared with those treated with surgery (12.0 versus 29.5 days, respectively; p=0.02).29 Da Luz Moreira et al.30 reported that percutaneous drainage successfully delayed elective surgery in 65% of patients, and that this conservative approach resulted in higher quality-adjusted life-years and was more cost-effective than initial surgery, despite the risk of failure and septic complications. However, one study found that outcomes were better in patients who underwent immediate surgery, compared to those who underwent percutaneous drainage followed by rescue surgery.31 It should also be noted that patients undergoing surgical resection were significantly less likely to develop abscess recurrence than those treated with antibiotics alone or percutaneous drainage over a mean follow-up of 3.75 years; however, non-operative therapy prevented subsequent surgery in half of patients.32
More recently, several studies have indicated that percutaneous drainage followed by resumption of medical therapy may reduce the need for surgery. One retrospective review of 25 cases of patients 20 years or younger with CD-associated abscesses found that percutaneous drainage was clinically successful (no surgery within 1 year, or surgical resection with no residual abscess detected) in 76% of cases.33 The authors also reported that early resumption of immunosuppressive therapy (within 8 weeks of drainage) was significantly associated with clinical success and avoidance of bowel resection.33
Percutaneous drainage alone has been shown to be less successful in patients with more severe disease, indicated by a thicker bowel wall, greater length of affected bowel, greater bowel dilation, and abscess size of >6 cm; and in patients with previous bowel resection or more advanced age (>50 years).33,34 Perl et al.34 stressed that even if an abscess resolves successfully without surgery, the fact that it occurred at all is indicative of severe disease activity, which will potentially demand bowel resection in the future.
One study found that percutaneous drainage alone was less likely to be successful in children if the abscess developed while the patient was receiving immunosuppressive therapy.24 However, other studies reported that biologic use at the time of abscess identification was not associated with a risk of future surgery,34 and a retrospective analysis of data from Belgium showed that treatment with anti-TNF agents was significantly associated with successful conservative management of CD-related abscesses, particularly in younger patients without previous bowel resection.35
ANTI-TNF TREATMENT OF CROHN’S DISEASE DOES NOT PREDISPOSE PATIENTS TO ABSCESS DEVELOPMENT
Anti-TNF agents are immunosuppressive, which raises the question of whether they might reduce host defences against abscess development. However, the evidence to date does not support this hypothesis. In the ACCENT II study, 15% of patients receiving infliximab maintenance therapy developed an abscess compared with 19% of patients receiving placebo (p=0.526), indicating that abscess development in patients with fistulising CD was not dependent on cumulative infliximab exposure.36
In contrast, systemic corticosteroid therapy was shown to be associated with an increased risk of developing an intra-abdominal or pelvic abscess in non-operated patients with perforating CD (adjusted odds ratio [OR]: 9.03; 95% confidence interval: 2.40–33.98).35,37
ANTI-TNF TREATMENT AS PART OF CONSERVATIVE MANAGEMENT
Until recently, there was little data available regarding the use of immunosuppressive therapy during the treatment of CD-related abscesses.23 However, it was generally understood that if anti-TNF agents were to be used in the setting of intra-abdominal abscesses, concomitant antibiotics should be administered until sepsis has resolved.23 The use of anti-TNF agents in this setting is based on the assumption that abscesses arose from a diseased bowel, which needs continued treatment to encourage healing and prevent abscess recurrence.23
Over the last decade, accumulating data has suggested that anti-TNF therapy in combination with conservative management of sepsis is beneficial in the context of CD-associated spontaneous intra-abdominal abscess.21,22,35,38 Long term follow-up of patients from the multicentre, prospective, observational MICA study showed that after sepsis was controlled, adalimumab treatment was successful in 66% of patients at Week 48, with an intestinal resection-free survival of 76% at 4 years.22 Factors associated with failure of adalimumab were the presence of stricture and/or proximal small bowel dilation,22 and surgery should be considered at first-line in these patients. A retrospective study in the USA reported that the use of anti-TNF therapy, versus no therapy, statistically significantly reduced the risk of abscess recurrence in patients who did not undergo surgery (p<0.001),21 and an analysis of data from Belgium similarly reported a significant association of anti-TNF treatment with successful conservative management of CD-related abscess.35 A retrospective chart review of patients with CD-associated intra-abdominal abscess treated with broad-spectrum antibiotics (with or without percutaneous drainage) and anti-TNF therapy found that none of the 12 patients experienced an increase in abscess volume, development of enterocutaneous fistula, soft tissue infection, or a need for emergency surgery, over a mean follow-up of approximately 3 years.38
ANTI-TNF TREATMENT PRIOR TO SURGICAL RESECTION
Similar to questions regarding the immunosuppressive nature of anti-TNFs and abscess development, there have been concerns about whether the peri-operative use of anti-TNFs might increase the rate of post-operative complications in patients with CD who require surgery.25,39 ECCO guidelines indicate that anti-TNFs have been shown to increase the risk of infectious complications in some, but not all, studies.25
One study assessed post-operative outcomes in patients with IBD who had detectable (≥0.98 μg/mL) serum levels of anti-TNF agents within 7 days of surgery.39 In patients with ulcerative colitis, the rate of complications was similar between those patients with detectable and undetectable levels of anti-TNFs. However, in patients with CD, there was a trend towards higher rates of post-operative complications in patients with detectable levels of anti-TNFs, compared to those with undetectable levels. Furthermore, patients with CD with anti-TNF serum levels ≥3 μg/mL showed a significantly higher risk of post-operative morbidity and infectious complications (OR: 2.5 and OR: 3.0, respectively; both p=0.03) compared with patients with levels <3 μg/mL. The authors concluded that increasing pre-operative serum anti-TNF levels were associated with adverse post-operative outcomes in patients with CD, but not those with ulcerative colitis.39
Similarly, Appau et al.40 reported that infliximab use within 3 months prior to ileocolonic resection was significantly associated with 30-day post-operative readmission (p=0.045), sepsis (p=0.027), and intra-abdominal abscess development (p=0.005). Data indicated that diverting stoma may protect against these complications.40 Syed et al.41 reported that the use of anti-TNF therapy in patients with CD during the 2 months prior to any intra-abdominal surgery was independently associated with increased post-operative complications (OR: 2.43; p=0.05 for infectious complications and OR: 1.96; p=0.10 for surgical site complications). However, major post-operative and intra-abdominal septic complications were similar between patients with and without anti-TNF use in the pre-operative period.41
Conversely, several studies have found that pre-operative use of anti-TNFs was not associated with an increased rate of post-operative complications42 or further CD-related surgery.16 A systemic review and meta-analysis of studies conducted up to January 2019 found no evidence that anti-TNF was a risk factor for post-operative surgical site infection in patients with IBD when the last infusion was administered 4 or more weeks prior to surgery.43 In addition, no significant difference in post-operative complications was found between patients receiving their last infusion within 4 weeks of surgery versus those receiving it ≥4 weeks before surgery, with the exception of subgroups from a single study (one out of 27 studies).43
A comparative retrospective chart review of adults with CD who underwent abdominal surgery found that administration of an anti-TNF within 12 weeks of surgery resulted in similar rates of post-operative surgical site infection and hospital readmission rates compared to the administration of ustekinumab, a monoclonal antibody specific to IL-12 and IL-23, in the same time period (differences of p=0.61 and p=0.14, respectively). However, ustekinumab was associated with a higher rate of returning to the operating room for any reason, compared with anti-TNFs (16% versus 5%; p=0.01).44
ANTI-TNF TREATMENT AFTER SURGICAL RESECTION
In patients with CD who develop post-operative abscesses, immunosuppressive therapy is generally withheld until the abscesses have healed.45 However, in patients with spontaneous abscesses, which require surgical treatment, post-operative anti-TNF therapy is considered a highly effective method to prevent bscess recurrence.21,45
CONCLUSION
Overall, an initially conservative approach (antibiotics with or without percutaneous drainage) for the management of CD-related intra-abdominal abscess is recommended by many guidelines,14,25,26 with the aim of delaying surgical resection and the associated risk of morbidity and stoma.26 This should be followed by diagnostic evaluation to determine whether surgery or therapy should represent the next step in management for each patient, based on the presence of stricture and whether abscesses developed during immunosuppressive therapy.45 In patients with free peritonitis,26 localised abscess,26,27 stricture,22 or proximal small bowel dilation,22 surgery may be more suitable as the initial management approach.
The literature to date indicates that anti-TNF therapy is unlikely to increase the risk of abscess development in patients with CD,36 and supports the use of anti-TNFs in patients with CD-related intra-abdominal abscess once sepsis has been controlled,22 suggesting that these agents may protect against recurrence of intra-abdominal penetrating disease.21








