Abstract
Recent epidemiological data gathered by the Centers for Disease Control and Prevention (CDC) suggest that colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the USA. Clinicians currently use five types of test to screen for CRC. Two of these five types, the DNA stool test and the faecal occult blood test, are non-invasive. The DNA stool test successfully detects both advanced neoplasias and non-advanced adenomas with more sensitivity than the faecal occult blood test. However, data suggest that it also generates more false-positive results. There is only one DNA stool test that has been approved by the U.S. Food and Drug administration (FDA): the approved Cologuard® (Exact Sciences, Madison, Wisconsin, USA) test. This test screens for nine different DNA biomarkers, one haemoglobin biomarker, and one β-actin. This article is a literature review of research on faecal DNA biomarkers conducted in the past 5 years from four large databases. Key findings include the ability to reach a sensitivity as high as 98% to detect abnormalities in the colon using a multi-target stool DNA-based assay. In comparison, the Cologuard offers 92% sensitivity and 87% specificity for all stages of CRC. Testing DNA biomarkers can serve as an adequate screen for cancer and adenomas in average-risk adults. Areas for further research include implementing studies to compare long-term health consequences for patients who receive colonoscopies versus DNA stool tests, finding ways to improve both the sensitivity and specificity of screening tests, and finding ways to improve the detection of those biomarkers most associated with CRC, including microRNA detection in the marking panel.
INTRODUCTION
According to the Centers for Disease Control and Prevention (CDC), colorectal cancer (CRC) is currently the second leading cause of cancer-related deaths in the USA.1 In 2013, approximately 71,000 men and 65,000 women were diagnosed with CRC.1 Primary care providers use five types of test to screen for CRC: two faecal occult blood tests (the guaiac faecal occult blood test and the faecal immunochemical test [FIT]), a DNA stool test (also referred to as the FIT-DNA), sigmoidoscopy, colonoscopy, and virtual colonoscopy.2 Unfortunately, many symptoms of CRC, such as a change in bowel habits, blood in the stool, narrow stools, weight loss, fatigue, and vomiting, only manifest once the cancer has approached late stages. Almost half of CRC incidences are detected at late stages.3 Therefore, early screening, accurate detection, and early intervention are all key to improved outcomes and decreased mortality.
Early detection of CRC is crucial to treatment and survival. The most reliable way to detect early CRC is through colonoscopy.4 Colonoscopy offers high accuracy and allows for longer time between screening but also has numerous disadvantages, including an extensive bowel preparation process, risks due to general anaesthesia, risk of bowel perforation, and length of the procedure.4 The U.S. Preventive Services Task Force recommends that all adults have a colonoscopy for screening purposes, beginning at 50 years old, and that they undergo a repeat colonoscopy every 10 years if the results are normal, until the age of 75 years.4
In contrast, faecal occult blood tests and DNA stool tests offer a non-invasive screening alternative that involves no preparation and can be completed at home.4 The development of CRC is a multistep process that begins with the normal mucosa of the large intestine mutating into abnormal lesions.5 This causes a continuous shedding of mutated cells that are then excreted into the faeces.5 Since human DNA is stable in faeces, it can be separated out and analysed for tumour-associated alterations.5 Many tests have been conducted to identify the mutations most associated with CRC; however, there is currently just one DNA stool test approved by the U.S. Food and Drug Administration (FDA): the Cologuard® (Exact Sciences, Madison, Wisconsin, USA). The National Cancer Institute determined that Cologuard can discern microscopic blood as well as nine DNA biomarkers that code for three different genes (NDRG4, BMP3, and KRAS). These genes have been linked to both CRC and adenomas, which are precancerous but advanced growths found in the gut. As cells pass through a patient’s colon and rectum, DNA from these cells shed and bind together in the stool. A specialised computer program can successfully analyse these cells and categorise them into positive or negative findings. Patients who are given a positive result are recommended to proceed with a colonoscopy.5
In a substantial study, it was demonstrated that in patients at average risk of developing CRC with no cancer-suggestive symptoms at the time, Cologuard could detect both more adenomas and more cancerous lesions than the FIT test.5 In other words, Cologuard was more sensitive than the FIT test. It should be noted, however, that Cologuard produced more false-positive results than the FIT test.5 Cologuard tests for seven DNA mutation biomarkers (KRAS) and two DNA methylation biomarkers (NDRG4 and BMP3), as well as one haemoglobin biomarker and one β-actin marker.6 Cologuard is currently covered under Traditional Medicare and Medicare Advantage plans for patients between the ages of 50 and 85 years who show no signs or symptoms of CRC and are at an average risk of developing CRC.7 Medicaid coverage varies by state, and private insurance policies vary greatly. The out-of-pocket cost for one kit is $649.6
While the future for DNA stool tests is promising, there is a strong need for further research into DNA biomarkers beyond the nine currently used in Cologuard, as well as for research that validates the sensitivity and specificity of these biomarkers, particularly in detecting adenomas. In the current article, the authors investigated the following population, intervention, control, and outcomes (PICO) clinical question: Is faecal DNA testing accurate in screening for CRC? The following four online databases were used: PubMed, Scopus, Web of Science, and Cumulative Index to Nursing and Allied Health Literature. A review was conducted of the literature spanning the past 5 years from 2012 to January 2017.
METHODS
Search Strategies and Key Term Definitions
PubMed was the first database searched. The search began with ‘colorectal cancer screening’ and results were further narrowed by adding the additional keywords ‘fecal DNA biomarkers’ and ‘noninvasive’. This yielded a total of 17 suitable articles from the PubMed database. Secondly, those same search terms were used in Scopus, which resulted in 11 articles. Thirdly, a search was conducted in Web of Science using the same key terms, which yielded 10 articles. Finally, a search search of the Cumulative Index to Nursing and Allied Health Literature resulted in no further articles after all the key terms were entered. This review of the literature was conducted on 11th February 2017.
The authors maintained the following inclusion criteria: articles published in English, published within the past 5 years, articles must pertain to faecal DNA biomarker testing rather than serum DNA, and articles must pertain to DNA testing rather than RNA. The terms ‘faecal DNA biomarkers’ and ‘non-invasive’ can be defined as follows: a faecal DNA biomarker is a biological DNA molecule found in faecal matter that is a sign of a normal or abnormal process, or of a condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease or condition. Biomarkers are also called molecular markers and signature molecules.8 Non-invasive is defined as: “A procedure that does not require inserting an instrument through the skin or into a body opening.”9
Organising the Evidence and Assessing Evidence Quality
To organise these articles, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram was used (Figure 1). This tool illustrates the stages of research conducted in a systematic review.10 At the end of the searches from all four databases, 38 articles were identified. Nine articles were removed because they were duplicates (Figure 1). One article was not accessible through electronic means and was therefore excluded. Nineteen articles were removed due to irrelevance; many articles in this group were investigating other types of cancers, blood screening tests, and RNA testing. The nine articles that remained focussed specifically on the effectiveness of faecal DNA testing in the screening of CRC. These nine articles were evaluated using the Grading of Recommendations Assessment, Development, and Evaluation method. This is a system used for rating the quality of research evidence and considers the study design and magnitude of effect, along with the risk and presence of bias, imprecision, inconsistency, and indirectness. It assigns one of four levels of quality: high, medium, low, and very low (Table 1).11
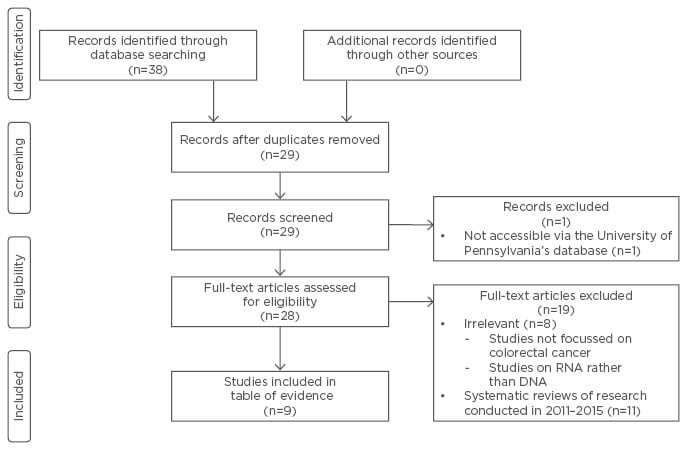
Figure 1: Preferred reporting items for systematic reviews and meta-analyses.
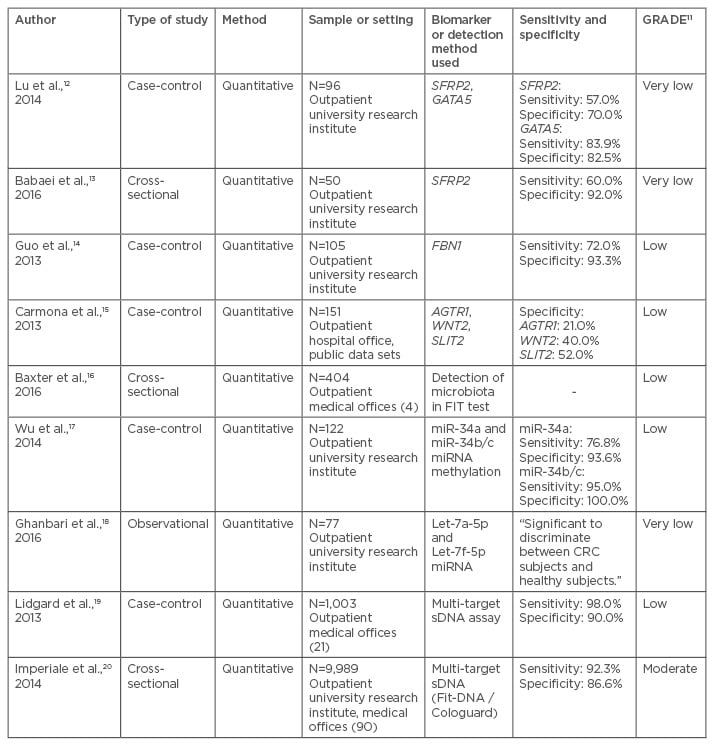
Table 1: Grading of recommendations assessment, development, and evaluation system applied to articles pertaining to faecal DNA testing in colorectal cancer screening.
AGTR1: angiotensin II receptor type 1; FBN1: fibrillin 1; FIT: faecal immunochemical test; GATA5: GATA binding protein 5; GRADE: Grading of Recommendations Assessment, Development, and Evaluation; miRNA: microRNA; sDNA: stool DNA; SFRP2: secreted frizzled-related protein 2 gene; SLIT2: Slit homolog 2 protein; WNT2: WNT family member 2.
RESULTS AND COMMON THEMES
Summary of Studies found in the Literature Review
Given the vast range of possibilities in the field of genetics and epigenetics, the studies analysed each focussed on different DNA markers (Table 1). Many focussed on determining the specificity of a single DNA mutation in detecting CRC.12-14 Lu et al.12 and Babaei et al.13 both studied the SFRP2. Carmona et al.15 looked at three genes: AGTR1, WNT2, and SLIT2, while Baxter et al.16 conducted a study that isolated the 16S ribosomal RNA gene. Other studies focussed on epigenetics, specifically the methylation of promoter genes, which causes the potential for the hyperexpression or silencing of genes.17,18 Several hypermethylated genes have been linked with CRC; Wu et al.17 specifically looked at microRNA promoters in this context. Lastly, two articles tested stools for both genetic and epigenetic mutations, which is how Cologuard screens for CRC;19,20 Lidgard et al.19 and Imperiale et al.20 both used a multi-target stool DNA (sDNA) assay to measure sDNA markers as well as methylation. All studies differed regarding sample size, DNA markers, control groups, and findings. However, analysing the sensitivity and specificity of different biomarkers is beneficial in drawing conclusions about the usefulness of these tests relative to one another.
Sensitivity and Specificity of Biomarkers
Of the nine studies found through the literature search, seven studies researched the sensitivity and specificity of stool biomarkers. Sensitivity is the extent to which a test can correctly identify a positive result (true-positive) and specificity is the extent to which a test can correctly identify a negative result (true-negative). A test with low sensitivity would produce more false-negatives and a test with low specificity would produce more false-positives. The sensitivities of the tests in the studies identified ranged from 57–98% (Table 1). Lidgard et al.19 found the highest sensitivity (98%) using a multi-target sDNA assay to measure β-actin (a marker of total human DNA), mutant KRAS, aberrantly methylated BMP3 and NDRG4, and faecal haemoglobin. It is possible that the high sensitivity was due to their test being an assay of multiple biomarkers for CRC. The study with the lowest sensitivity was Lu et al.,12 which tested the biomarker SFRP2 (57%). Babaei et al.13 also studied SFRP2 in stool samples and found a similar specificity of 60%. For specificity, the tests ranged from 21–100% (Table 1). Wu et al.17 studied the methylation status of miR-34a and miR-34b/c promoter in CRC patients’ stool samples and found the specificity of miR-34b/c was 100%. Carmona et al.15 studied three genes in relation to detecting CRC in patients’ stool: AGTR1, which had a specificity of 21%; WNT2, which had a specificity of 40%; and SLIT2, with a specificity of 52%.
CONCLUSION
This research is extremely relevant to many healthcare providers since it pertains to common screening tools. While providers currently just have one FDA-approved option (Cologuard), genetic detection of cancer risk continues to be an area of research and development. The Cologuard test is indicated in the screening of all adults ≥50 years old who are at an average risk for developing CRC. It is contraindicated in patients with a history of any form of cancer, if they had a previous positive result from any CRC screening method, have a family history of CRC, or have an inflammatory bowel disease.6 Although this is an approved screening method that offers convenience and no direct risks to the colon, not all providers have embraced this new recommendation and the CDC continues to advise adults to have colonoscopies.2 Hopefully, with continued research and improvements in the testing methods, this screening option will become more available and accurate in detecting CRC in adults.
Need for Further Research
There is still a great need for research in this area. To date, there have been no randomised controlled trials that compare different screening tests, such as colonoscopy and DNA stool-based tests, to one another; only studies that test the reliability, sensitivity, and specificity of specific screening tests have been performed. Because of this, it is difficult to know which tests are the most effective. Analysing long-term outcomes, including morbidity and mortality, is necessary to assess the role of multi-target sDNA testing. Another area for future research is on issues related to testing intervals, patient acceptance, compliance of screening guidelines, and barriers that prevent individuals from being screened for CRC. It is unclear if patient preference for non-invasive screening tools along with more effective methods of non-invasive screening will one day diminish the importance of the use of colonoscopies in the average-risk adult. Additionally, mRNA and microRNA are areas of current research that offer potential for highly specific and sensitive CRC screening. While more research is needed in several areas, the role of multi-target sDNA testing is continuously advancing and offers an affordable, convenient, and safe option for adults who are at an average risk for developing CRC.








