INTRODUCTION
In parallel to the blood vascular system, an extensive network of lymphatic vessels circulates throughout the entire body. The lymphatic system plays an important role in maintaining tissue fluid homeostasis, immune cell trafficking, and in particular facilitates dietary lipid uptake in the intestine.1 Paneth cells are a part of the intestinal innate immune system and have a central role in regulation of intestinal angiogenesis.2 It has been previously reported that a decrease in intestinal vascularisation and number of Paneth cells occurs alongside lymphangiogenesis in the absence of intestinal microflora.3 However, the association between Paneth cells and the regulation of lymphatic vascular development is currently unknown. The aim of this study was therefore to investigate the regulatory role of Paneth cells in the development of intestinal and mesenteric lymphangiogenesis and portal hypertension.
METHODS
The experiment involved inducing Paneth cell depletion in Math-1 Lox/Lox VillcreERT2 mice by injecting three consecutive doses of tamoxifen and performed partial portal vein ligation (PPVL) to induce portal hypertension. After 14 days, portal pressure was measured. Intestinal and mesenteric lymphatic vessels were assessed by immunohistochemistry using lymphatic vessel endothelial hyaluronic acid receptor 1 (Lyve-1) antibody. The lymphatic vessels were quantified using MetaMorph® (Molecular Devices, San Jose, California, USA) software to calculate pixel percentage ratio. Expression of genes involved in the regulation of lymphatic vessels was evaluated by RT2 Profiler™ PCR array (Qiagen, Hilden, Germany) in intestinal tissue. Intestinal organoids from controls and Paneth cell depleted mice (Figure 1) were exposed to various bacterial derived products. Proteomic analysis of conditioned media was performed using MaxQuant software to analyse differentially regulated proteins associated with lymphangiogenesis in the absence or presence of Paneth cells or in portal hypertension.
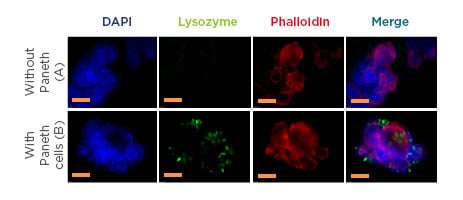
Figure 1: Organoids with (green) or without Paneth cells.
Representative immunofluorescent images of small intestinal organoids cultured from A) Paneth cell depleted mice small intestine B) control mice small intestine with Paneth cells. Nuclei (blue), Paneth cells (Green), F-actin (Red). F-actin is endogenously expressed and is stained with phalloidin, Paneth cells are detected using a polyclonal rabbit antibody lysozyme in combination with a secondary antibody Alexa Fluor®488 (Thermo Fisher Scientific, Waltham, Massachusetts, USA) F(ab’)2 fragment of goat anti-rabbit IgG. Nuclei are labelled with DAPI. Images of organoids were obtained using a Leica DMI4000 B (Leica Microsystems, Wetzlar, Germany) fluorescence system. The yellow line represents 100 µM and 50 µM magnification in both A and B, respectively. DAPI: 4`,6-diamidino-2-phenylindole.
RESULTS
Portal pressure was significantly attenuated in Paneth cell depleted mice compared to control mice after PPVL (n=11/group, 9.78±1.23 cm H2O versus 11.45±1.41 cm H2O, respectively; p<0.002). Depletion of Paneth cells resulted in a significantly decreased density of lymphatic vessels compared to controls as assessed by immunohistochemistry (n=5, pixel ratio), in the intestine (0.176%±0.12 versus 0.367%±0.15; p=0.01), and in the mesentery (0.160%±0.06 versus 0.404%±0.20; p=0.001). Quantitative PCR showed a decreased expression of genes involved in the regulation of lymphangiogenesis, including VEGF-C, VEGF-D, VEGF-A, Nrp2, Angpt-2, Tie-1, Tie-2, TGF-α, HGF, and CXCL-1 in Paneth cell depleted mice.
Moreover, the expression of specific markers of lymphangiogenesis, such as transcription factor Prox-1, growth factor VEGFR3, or protein FOXC2, were significantly decreased in Paneth cell depleted mice after PPVL. In the absence of Paneth cells, proteomic analyses showed a significant downregulation of several proteins involved in lymphatic vessel development and morphogenesis, as well as in lipid metabolism and transport processes.
CONCLUSION
In the absence of Paneth cells, portal hypertension was attenuated significantly in association with a decreased density of intestinal and mesenteric lymphatic vessel. These findings suggest that Paneth cells not only play an antimicrobial role in the intestine, but also contribute to the regulation of lymphatic vessels and portal pressure.








