BACKGROUND
Oesophageal carcinoma is the eighth most common malignancy worldwide and the seventh most common cause of death in men.1 Most patients are diagnosed with oesophageal cancer in the advanced stages, with approximately 20% of cases being resectable.1 Concurrent chemoradiotherapy (CCRT) is superior to radiotherapy alone for patients with locally advanced oesophageal cancer.2 Definitive CCRT is a well-established treatment for oesophageal cancer, but many patients do not achieve remission with CCRT alone. Although several clinical and molecular markers can be used to evaluate the response to CCRT, they are not highly predictive. Ridky et al.3 found that the correlation between the primary tumour and a two-dimensional cell culture was very low, whereas the similarity between a three-dimensional tissue environment and the primary tumour was higher than the mouse to human correlation.3 Patient-derived organoids have advantages in providing more physiologically relevant and predictive data for in vivo responses.
AIM
Our aim was to evaluate the predictive power of CCRT response using a primary three-dimensional cell (organoid) culture in oesophageal cancer.
METHODS
Patient-derived organoid culture was performed using tumour tissues acquired from patients with oesophageal cancer before they began treatment with CCRT. After culturing the tissues for 7 days, same-sized organoids were collected and treated with fluorouracil (5-FU) and 5Gy radiotherapy. After 6 days, the primary cultured cells were stained and fluorescent images were captured. The clinical response was assessed after the fourth cycle of CCRT. Clinical response was classified as complete remission (CR), partial remission (PR), or disease progression (PD).
RESULTS
A total of 27 oesophageal cancer patients were enrolled. The final success rate of the patient-derived organoid culture was 78% (21/27). The CCRT response in patient-derived organoids was evaluated in 21 cases. A total of 16 people were followed-up for >4 cycles of CCRT and were analysed. Clinical CR was observed in 10 patients and 6 showed clinical PR (n=4) or PD (n=2). Strong live activity (green fluorescent image) was noted in the control group, whereas extremely low live activity and strong dead activity (red fluorescent image) was observed in the CCRT group (Figure 1A). In the clinical setting, these patients achieved long-term CR. Strong live activity was noted in the control group and also in the CCRT group (Figure 1B). In the clinical setting, these patients showed initial PD. Live activity was noted in <10% of organoids in all patients with clinical CR and was observed in 30–40% of organoids in all patients with clinical PD. Live activity was noted in <20–30% of organoids in all patients with clinical PR. Among 16 patients, organoid-based CCRT response prediction was matched with clinical CCRT response in 15 patients (93%) and 1 case was unmatched.
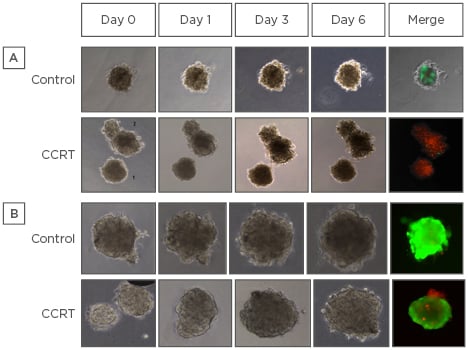
Figure 1: Concurrent chemoradiotherapy response in patient-derived primary organoids.
A) Strong live activity (green fluorescent image) was noted in the control group, whereas extremely low live activity and strong dead activity (red fluorescent image) were observed in the CCRT group. B) Strong live activity was noted in the control group and also in the CCRT group.
CCRT: concurrent chemoradiotherapy.
DISCUSSION
It took 2 weeks to evaluate the CCRT response in organoids from tissue acquirement. Furthermore, a high agreement between clinical response and response in organoids was observed. Therefore, the evaluation of CCRT response using patient-derived primary organoids will provide a good predictor of clinical CCRT response, making precision medicine one step closer. Investigation for the molecular similarity between primary tissue and patient-derived organoids will provide more concrete evidence for the usefulness of patient-derived organoids.








