INTRODUCTION
Irritable bowel syndrome (IBS) affects 10–15% of the world population. It is the most common cause of primary gastroenterology visits and affects twice as many females than males.1 IBS is characterised by visceral pain associated with alterations in bowel transit (either diarrhoea, constipation, or a mix of both). IBS greatly affects quality of life and is highly correlated with psychological comorbidities; 30–50% of patients with IBS report anxiety or depression.2 The pathophysiology of IBS is complex and differs among patients. It can include visceral hypersensitivity, increased paracellular permeability, and gut microbiota dysbiosis, classifying IBS as a pathology of the gut-brain axis.2 Among IBS risk factors, stress, in particular neonatal stress, increases both the incidence of the disease and the worsening of the symptoms.3 Prenatal stress (PS), which induces gut microbiota dysbiosis, has recently been identified as a risk factor for anxiety and depression, both of which are IBS comorbidities.4,5 However, the causal link has not been established, and the authors hypothesised that PS in mice would predispose the adult offspring to visceral hypersensitivity and intestinal homeostasis disruption, as observed in IBS.
METHODS
PS was induced in C57bl/6 mice by using a bright light (coupled to restriction) for 30 minutes three times a day between Days 13 and 18 of gestation. Visceral hypersensitivity to colorectal distention was assessed in male (n=20) and female (n=20) 8-week-old offspring by recording abdominal contractions in response to pressures of colorectal distension at 15, 30, 45, and 60 mmHg. The paracellular cellular permeability of the offspring was measured by 4 kDa fluoresce in isothiocyanate–dextran (FiTC)-dextran (10 mg/mice) gavages, followed by fluorescence measurement in the plasma 4 hours later. Cxcl2, Tgfb, Ccl5, Reg3g, Muc2, Occln, Ttf3, Mmp7, Penk, and Ifng colonic expressions were monitored by real-time quantitative reverse transcription-PCR. The fecal microbiota composition was assessed by the MiSeq-based microbial taxonomic method and its organisation as a biofilm was evaluated by 16S RNA FISH staining.
RESULTS
In the offspring of both sexes, PS induced hypersensitivity to colorectal distensions expressed as the area under the curve for the lowest (15–30 mmHg) and the highest (45–60 mmHg) pressures of distension (Figure 1).
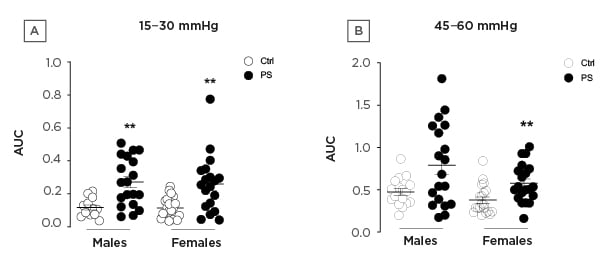
Figure 1: Area under the curve of the visceromotor response to colorectal distension of (A) 15–30 mmHg and (B) 45–60 mmHg in control (white circles) male (n=20) and female (n=20) and prenatal stress (black circles) offspring.
** p<0.01 compared to control.
AUC: area under the curve; Ctrl: control; PS: prenatal stress.
Female mice were significantly more hypersensitive to colorectal distension for both types of pressure, while the increased sensitivity in the male offspring was significant only for the lowest pressures of distension (Figure 1). Paracellular permeability and gene expression in the colon remained unaltered by PS in both males and females. PS mice gut microbiota analyses revealed a dysbiosis as well as an alteration of the biofilm organisation. Indeed, Akkermansia muciniphila was increased in stressed male and female mice, while Desulfovibrio spp and Lactobacillus animalis were decreased in males and females, respectively. The alteration of the gut microbiota biofilm in PS mice was marked by bacterial infiltrations in the sterile mucus layer.
Finally, spearman correlations showed the existence of an inverse correlation between the abundance of L. animalis in the female faeces and visceral hypersensitivity for the lowest (R= -0.6; p=0.006) and highest (R= -0.61; p=0.007) pressures of distension. The same correlation was found in males, but only for the highest pressures. (R= -0.6; p=0.005).
CONCLUSION
This study shows that PS is sufficient to induce visceral hypersensitivity, gut microbiota dysbiosis, and biofilm disruption in mice. Therefore, PS could represent an important priming event for the development of IBS in adulthood.








