BACKGROUND AND AIMS
A gluten-free diet is the only therapy that provides clinical and histological remission of coeliac disease. The detection of gluten immunogenic peptides (GIP) in faecal samples, only present if gluten is ingested, may constitute a useful tool to evaluate dietary adherence.
MATERIALS AND METHODS
This is an observational, retrospective, longitudinal, between-groups study design. The authors collected clinical data from patients who had GIP measurement during follow-up from January 2017–June 2021. A descriptive and comparative analysis was performed using SPSS software (IBM, Armonk, New York, USA).
The primary objective was to evaluate the clinical effectiveness of GIP measurement during follow-up of patients with coeliac disease. The secondary objective was to analyse the relation between GIP levels and demographic data (gender and age), laboratory results, and histopathology.
RESULTS
Of the 103 patients included, 182 faecal samples were obtained. The characteristics are summarised in Table 1. A total of 29 patients had positive levels of GIP; 12 of them normalised during follow-up (41.38%).
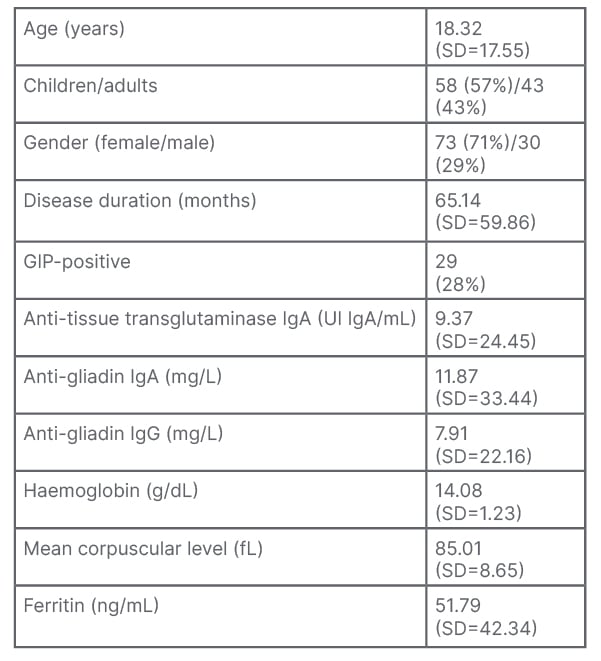
Table 1: Characteristics of patients included (N=103).
Quantitative variables are expressed as means and SD; qualitative variables are presented as total number of events and percentage.
GIP: gluten immunogenic peptides; SD: standard deviation.
No differences were found between haemogloblin, ferritin, mean corpuscular volume, or antibody levels, and the result (positive/negative) of GIP in the total samples, nor when analysing the samples of adults and children separately. Only anti-gliadin IgG antibodies were higher in GIP-positive patients (p=0.028). No differences were found between the result (positive/negative) of GIP and histology at diagnosis, gender, or age (adults/children). It is important to mention that 77 patients had negative anti-tissue transglutaminase levels and 21 of them (27.27%) had GIP in faeces.
The authors did not find differences in the result of GIP depending on age or disease duration. However, when paediatric patients were selected, positivity for GIPs increased with age (p=0.032).
CONCLUSION
GIPs are a useful tool in the follow-up of patients with coeliac disease to monitor dietary compliance, and may detect dietary transgression in subjects with negative antibodies. Antibody levels, age, and disease duration are not associated with positivity rates of GIP. Only adolescents in the paediatric group had more dietary transgressions according to detection of GIP.








