Meeting Summary
This article summarises a LivaNova-sponsored symposium entitled ‘The Power of Real-World Evidence with CORE-VNS’, delivered on 7th September 2024 as part of the 15th European Epilepsy Congress in Rome, Italy. The symposium explored real-world data from CORE-VNS, a global, prospective long-term study of patients receiving adjunctive Vagus Nerve Stimulation (VNS) TherapyTM for drug-resistant epilepsy (DRE), which represents one of the most comprehensive contemporary real-world clinical data sets in DRE. The presentations provided an overview of the strengths, limitations, and executional requirements of large-scale real-world evidence studies in DRE. In addition, the impact of adjunctive VNS Therapy on both seizure and non-seizure outcomes in DRE within the European healthcare setting was explored.
In the symposium, two speakers closely involved in the conduct of the CORE-VNS study discussed insights from the study to date. Gaia Giannicola, Clinical Senior Project Manager and Program Manager for Neuromodulation at LivaNova, Milan, Italy, described the power of real-world data in the context of the CORE-VNS study, while Arjune Sen, Consultant Neurologist at the John Radcliffe Hospital in Oxford and Professor of Global Epilepsy at University of Oxford, UK, chaired the meeting and presented an interim analysis of European patients enrolled in the CORE-VNS study.
Introduction
Epilepsy affects approximately 50 million people around the world, and is associated with a high risk of disability, psychiatric comorbidity, social isolation, and premature death.1 More than one-third of people living with epilepsy continue to experience seizures, despite taking adequate and correctly dosed anti-seizure medications (ASM).2 DRE, defined as the failure of adequate trials of two anti-seizure medicine schedules to achieve sustained seizure freedom,1,3 may require additional non-pharmacological interventions such as surgery, dietary therapy, and/or neuromodulatory interventions.1,4
The VNS Therapy system is the most widely available form of neuromodulatory therapy for the treatment of DRE.4,5 Since its initial approval in 1994, the technology behind the VNS Therapy system has evolved, and it is indicated outside the USA for use as an adjunctive therapy in reducing the frequency of seizures in patients whose epileptic disorder is dominated by focal seizures (with or without secondary generalisation) or generalised seizures that are refractory to seizure medications.6 In the USA, the VNS Therapy system is indicated for use as an adjunctive therapy in reducing the frequency of seizures in patients 4 years of age and older with partial onset seizures that are refractory to antiepileptic medications.7
The real-life global experience of people with DRE treated with adjunctive VNS is currently being assessed in the ongoing CORE-VNS Post-Market Study.4,5 This study, which has enrolled more than 800 patients in total, provides the opportunity to analyse a broad set of clinical and healthcare utilisation endpoints in a large and diverse patient population, enabling extensive subpopulation analysis.
The study design for the CORE-VNS study has been published previously.4 In brief, the CORE-VNS study is an international, multicentre, prospective, observational, all-comers, post-market study enrolling people with DRE either receiving VNS Therapy for the first time or undergoing a VNS Therapy battery change.4 The presentations included at the LivaNova symposium described the design and baseline characteristics of patients participating in CORE-VNS, as well as the results of a recent interim analysis in a European subpopulation of the study.
The Power of Real-World Data: The CORE-VNS Study
Giannicola began her presentation by observing that the CORE-VNS study offers one of the most comprehensive contemporary real-world clinical data sets in DRE. A total of 827 patients consented to be part of this study at 61 sites in 16 countries across five continents, including 262 patients in 23 sites across the USA and Canada, 219 patients in 15 sites in six western European countries, and 346 patients in 23 sites across Asia-Oceania, Latin America, and the Middle East (data on file). Patients were assessed at baseline and then followed at 3, 6, 12, 24, and 36 months after enrolment.4,5
Endpoints analysed in the CORE-VNS study include seizure-associated outcomes comprising traditional measures of clinical benefit such as changes in seizure frequency and severity, as well as non-seizure outcomes including measures of sleep quality, quality of life, ASM use, and healthcare resource utilisation data. In addition, safety data collected include all deaths, adverse events (AE) related to VNS Therapy, and device deficiencies (data on file).4,5
In order to convey the value of real-world evidence, Giannicola noted that health outcomes in clinical practice are multifactorial, with many contributing elements. She provided data to show that the relative influence of the five major factors impacting health outcomes may be estimated as follows: social circumstances (15–40%), environmental and physical influences (5–20%), behaviour (30–50%), genetics (20–30%), and medical care (10–20%).8 Real-world evidence enhances our understanding of treatment effectiveness, safety, and outcomes in routine clinical practice, beyond the controlled environment of clinical trials. In particular, real-world evidence bridges the gap between clinical research and everyday healthcare, driving more informed, effective, and patient-centred solutions.
As a real-world study, CORE-VNS provides extensive long-term data that can offer valuable insights to guide various stakeholders, including healthcare providers, regulatory bodies, and insurance providers regarding the use of VNS Therapy. In addition, it offers long-term health outcome data associated with the use of different VNS Therapy features (such as Scheduled Programming or AutoStimulation) across a global landscape. Furthermore, the large sample size and broad inclusion criteria allow for broad applicability to everyday clinical practice. This inclusivity also facilitates in-depth subpopulation analyses, including an evaluation of the performance of VNS Therapy in groups that are typically more challenging to recruit for focused research studies.
Giannicola commented that it was only possible to carry out the CORE-VNS study due to the hard work of multiple key players all around the world. At each of the 61 clinical sites, the study teams, study nurses, study coordinators, and investigators are all jointly responsible for the conduct of the study in accordance with the clinical protocol, good clinical practice, and applicable regulatory requirements. Other key players on the LivaNova side include the clinical data management team, biostatisticians, programming experts, clinical monitors, safety specialists, and the clinical project management team.
CORE-VNS began with the first patient enrolment in 2018. The last patient was initiated in 2021, and the study is now in the close-out phase, with the last patient’s last visit expected by the end of 2024. The study relies on a combination of planning, executing, monitoring and controlling, and communication to ensure that it is delivered efficiently, accurately, and in a timely manner. In addition to standard data management activities, a strong data management strategy was developed in the CORE-VNS study with a focus on the review of seizures based on the 2017 International League Against Epilepsy (ILAE) classification.9 This includes, but is not limited to, the verification of consistent records of seizure frequency and type across visits, as well as consistency in seizure types, epilepsy type, and epilepsy syndromes captured. The data require careful monitoring and controlling through multiple data review cycles, resulting in data review meetings between clinical sites, medical affairs, and clinical affairs. Effective communication is ensured by tight collaboration between clinical sites, the clinical project management team, clinical monitors, statistics and data management experts, and the medical affairs team.
The large scale of the study and the dedication of all players to reach high data quality results is demonstrated by the numbers quoted by Giannicola. For example, more than 25,000 queries and edit checks have been resolved, and 4,052 seizure forms have been reviewed. In total, 3,279 patient follow-up visits have been conducted, 379 monitoring visits have been accommodated, and 252 patients with suspected genetic epilepsy have been reviewed from a clinical and genetic testing point of view by a special working group composed of paediatricians and genetics experts.
Giannicola reported that a total of 819 patients, out of the 827 who consented to be part of the study, met the eligibility criteria. Approximately 40% of the patients were under 18 years of age at implant, and a median of six ASMs had been trialled at baseline (range: 2–20). The median (range) time between epilepsy diagnosis and consent was 10 (0–62.5) years in the first-implant cohort. Cognitive status was impaired in 70.5% of the population, and the most common epilepsy type was focal (47.7%), followed by combined (34.2%) and generalised (16.1%) epilepsy. The aetiology of epilepsy was unknown in 41.2% of patients, with structural aetiology being the most commonly known type (33.3%), followed by genetic (17.0%), infectious (6.2%), immune (1.8%), and metabolic (0.5%) aetiologies. Data were also collected on 252 patients who had undergone genetic testing before baseline. On the basis of this dataset, Giannicola noted that CORE-VNS is on track to be one of the most comprehensive contemporary real-world clinical datasets available in DRE to date.
Giannicola concluded her presentation by reminding the audience that patients in CORE-VNS are affected by severe DRE and had inadequate seizure control despite a median of six ASMs prior to VNS Therapy. A large cross-functional team active across the globe is continuously monitoring and sampling data quality and entry to ensure highest quality results. Giannicola noted that although real-world evidence cannot substitute for randomised controlled trials (RCT), CORE-VNS provides insights to questions which cannot be answered by prior RCTs with VNS Therapy. The last patient will have their last visit at the end of 2024, upon which the study will be closed-out and multiple further analyses will begin.
CORE-VNS: Contemporary VNS Therapy Outcomes of People with Drug-Resistant Epilepsy in Europe
Sen followed Giannicola’s talk on the CORE-VNS study design and baseline characteristics with a presentation of the results of an interim analysis of the CORE-VNS study conducted among a subset of patients in Europe.
Of 338 patients who signed the informed consent and met the eligibility criteria, 11 did not have a record of VNS implant, leaving a total of 327 patients in the modified safety population (mSAF), a subset of subjects, with a signed informed consent and who met all eligibility criteria, who went through a VNS Therapy implant procedure (successful and unsuccessful). Among patients in the mSAF, 226 had their first implant during the trial, 100 underwent reimplantation, and one patient had an aborted implantation due to an AE. Unless otherwise stated, the analyses below refer to the first-implant population of 226 patients in the mSAF.
Patients included in the ‘European’ population were recruited in Israel (30%), the UK (27%), Belgium (16%), Italy (9%), the Netherlands (7%), Poland (6%), and Austria (5%). The mean (standard deviation) age at time of informed consent was 27.6 (17.3) years, and 65.4% of patients were aged ≥18 years at informed consent. Overall, 170 of 338 patients (50.3%) were female.
Among patients receiving their first VNS Therapy implant (n=226), the median (range) interval between diagnosis of epilepsy and informed consent was 10.8 (0.5–58) years, which highlights the considerable length of time that patients have to wait to receive VNS therapy. Individuals in this population had received a median (range) of seven (3–16) prior ASMs before being enrolled in the registry, suggesting that this is a highly refractory group. The number of prior ASMs is slightly higher than in the global population, potentially as a result of the greater drug availability. Focal epilepsy was the most common epilepsy type in this group (54%), followed by combined (30%) and generalised (14%) disease. Overall, 41% of first-implanted patients had epilepsy of an unknown aetiology, with 34%, 20%, 4%, and 1% having structural, genetic, infectious, and immune aetiologies, respectively. Just over one-third (36.7%) of first-implanted patients had normal cognitive status, with 18.1% experiencing severe impairment, 25.2% moderate impairment, and 19.9% living with minimal cognitive impairment. This population is therefore truly reflective of real-world clinical practice, unlike RCTs, where the study population may be enriched according to type of epilepsy or cognitive status.
Analysis of seizure frequency among first-implanted patients in the mSAF population revealed a reduction in seizure frequency at the end of the study compared with baseline for all types of epilepsy (reduction in seizure frequency from baseline at 36 months was 60.2% for all seizures [n=157 at 36 months], 57.8% for focal seizures [n=135 at 36 months], and 74.4% for generalised seizures [n=37 at 36 months]; Figure 1). The reduction in seizure frequency increased with time for all seizure types, with a notable reduction evident early in the study. This is consistent with other neuromodulatory therapies, which show that seizure frequency decreases with increasing duration of neuromodulation, and that the effect on all seizures is observable early after implantation. Sen cautioned that this is an interim analysis only and does not include 36-month data for all participants; although he noted that the interim analysis does seem to suggest a positive impact on seizures, irrespective of type.
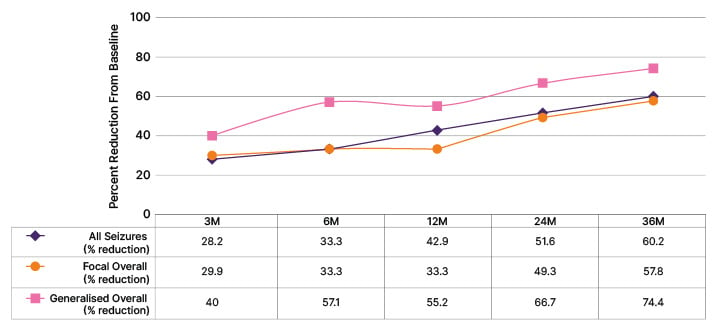
Figure 1: Median percent reduction in seizure frequency (interim data).
VNS implant-naïve patients. ASM changes were allowed during the follow-up period (LivaNova, Data on File).
ASM: anti-seizure medication; M: month.
As well as reduction in seizure frequency, there was a reduction in seizure counts over the previous 3 months between baseline (67 seizures; n=224) and 36 months (20 seizures; n=169), with some improvement noticeable by 3 months (37.5 seizures; n=190). This again suggests that VNS Therapy has a positive impact on the number of seizures.
Furthermore, disaggregation of the data reveals a reduction in seizure frequency for focal impaired awareness–motor seizures (median [95% CI] reduction of 67% [50–86.1] at 36 months [n=76] versus baseline), focal to bilateral tonic-clonic seizures (100% [58.3–100] reduction at 36 months [n=35] versus baseline), and generalised tonic-clonic seizures (73.2% [10–73.2] reduction at 36 months [n=24] versus baseline), although the wide confidence intervals for these datapoints should be noted.
The proportion of patients achieving >50% reduction in seizure rate increased progressively over the 36-month follow-up for all seizures, focal-onset seizures, focal to bilateral tonic-clonic seizures, generalised onset seizures, and generalised tonic-clonic seizures, notwithstanding the comparatively low number of patients in the 36-month dataset. Sen drew the audience’s attention to the relatively high proportion of patients achieving a greater than 50% reduction in number of seizures (Figure 2).
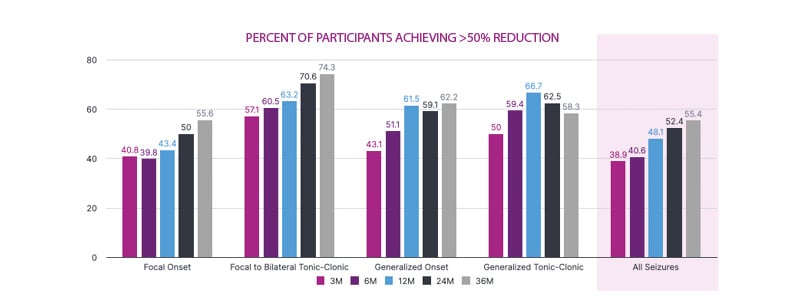
Figure 2: Effectiveness: Seizure responder rate (interim data).
VNS implant-naïve participants. LivaNova Data on File.
M: month.
Of 157 patients for whom 36-month response data were available, 10.2% achieved a 100% seizure-frequency reduction at 36 months, while 33.8% achieved ≥80% improvement, and 21.6% achieved ≥50 to <80% improvement in seizure rate at 36 months.
Because CORE-VNS tracks seizures by their specific type according to 2017 ILAE classification, it is important to assess whether seizure semiology changes in the course of VNS Therapy, resulting in the reporting of seizure types not reported at baseline. Sen presented an interim analysis showing that reporting of generalised seizure types not reported at baseline is very infrequent and occurs in less than 4% of patients. The picture is different for some focal seizure types. Approximately 20% of patients reported a specific focal seizure type in the course of VNS Therapy, which was not reported at baseline. The reason for this finding is not yet clear, although Sen speculated that it may represent natural seizure evolution of certain epilepsy types and/or VNS-associated changes in focal seizure propagation and therefore severity.
As well as seizure rate and counts, it is also important to consider the quality of life of patients with DRE. The percentage of patients reporting a very good quality of life (“could hardly be better”) appeared to increase between baseline and 36 months, while the percentage of patients with a very poor quality of life (“could hardly be worse”) decreased from baseline to 36 months (Figure 3).
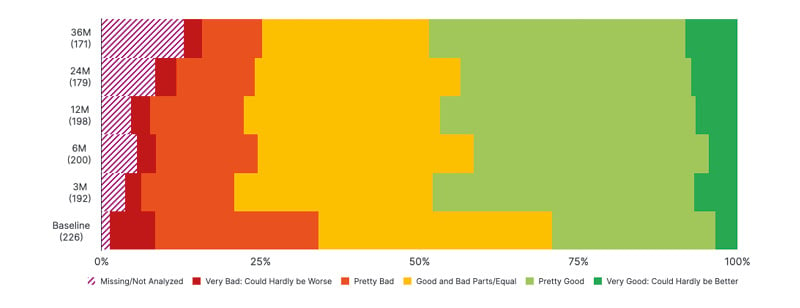
Figure 3: Summary of quality of life (first-implanted patients; percent of patients reporting; interim data).
LivaNova Data on File.
M: month.
In addition, there appears to be a reduction between baseline and 36 months in the percentage of patients reporting that the frequency of their seizures has an impact on their quality of life, as well as a reduction in the percentage of patients who report that the difficulty or intensity of their seizures impacts their quality of life, or that their ASM affects their quality of life. Again, Sen noted that these are interim data with further work needed but commented that this might be due to the increasing availability of new treatments for epilepsy that have improved side effect profiles, although he noted that newer treatments can adversely affect mood.
Across the European study, there were 13 AEs that led to study termination. This included 11 serious AEs and six deaths (one suicide, two cases of drowning, one subdural haematoma due to seizure-related fall, one sudden unexpected death in epilepsy, and one case of propofol infusion syndrome). Explantation of the VNS Therapy device occurred in eight patients. The treatment-emergent AEs (TEAE) recorded were consistent with previous experience of VNS Therapy, with 34.6% of 327 patients experiencing at least one TEAE. Dysphonia and dyspnoea were the most common TEAEs, reported in 11.6% and 5.8% of patients, respectively. Other TEAEs of interest included neck pain (4.0% of patients), implant site infection (1.8%), cardiac disorders (0.9%), vocal cord paralysis (0.6%), and sudden unexpected death in epilepsy (0.3%).
Sen concluded by reiterating that this presentation represented an interim analysis and that the final results may differ. Other limitations include the fact that CORE-VNS is an open-label registry, and as such is unblinded and has no control arm, while other anti-seizure treatments (for example, ASMs) could be added, withdrawn, or altered during treatment with VNS Therapy. In addition, missing data were not imputed in this interim analysis, so the bias arising from drop-outs and missing data has not been accounted for. Sen noted, however, that these limitations should also potentially be considered as strengths as they demonstrate that the study reflects real clinical practice, with data that may be more applicable to the everyday experience of patients and physicians.
Conclusion
CORE-VNS is the largest multicentre global post-market registry of patients diagnosed with DRE and treated with the VNS Therapy system, and provides additional insights into the effects of VNS Therapy that could not be addressed by prior RCTs alone. The CORE-VNS data presented here offer crucial evidence to guide informed treatment decisions for patients with DRE.
More than half of the 338 European patients in CORE-VNS had focal epilepsies, and approximately one-third of patients were children. The majority of European patients included in CORE-VNS were from Israel and the UK. Not all patients have yet undergone the 36-month follow-up visit, with data from 169 of the 226 newly implanted patients available for analysis in this interim dataset. However, this interim analysis demonstrates that seizure frequency (of all seizures) was reduced at the 36-month visit by 60%, with the greatest reduction seen for generalised seizures, which were reduced by 74%. In addition, 55% of patients had experienced more than 50% seizure frequency reduction, and 34% had experienced more than 80% seizure frequency reduction by the 36-month visit. Finally, no unexpected AEs or safety concerns occurred at the point of interim analysis.
| Safety Information for VNS TherapyTM
Epilepsy (Non-US)—The VNS Therapy System is indicated for use as an adjunctive therapy in reducing the frequency of seizures in patients whose epileptic disorder is dominated by partial seizures (with or without secondary generalisation) or generalised seizures that are refractory to seizure medications. Incidence of adverse events following stimulation (>5%) were voice alteration, increased coughing, hoarseness, shortness of breath, sore throat and nausea. Infection is the most common complication of the surgical procedure. Please see important safety information at epilepsy.livanova.com/ous-safety-information. The complete copy of the VNS Therapy™ System Epilepsy Physician’s Manual available at epilepsy.livanova.com/manuals. |







