Meeting Summary
Susan Fox opened this satellite symposium at the 8th European Academy of Neurology (EAN) Congress with an overview of the concept of motor fluctuations (MF) in Parkinson’s disease (PD). She emphasised that levodopa remains the gold standard therapy for PD. However, MFs are one of the critical complications of levodopa therapy that affect many patients with advancing PD and, when diagnosed, represent a challenge in patient management. Alternative options are, therefore, needed to provide continuous dopaminergic stimulation while maximising the levodopa benefit. Despite different options, Angelo Antonini showed that neurologists often prefer to adjust levodopa dose rather than add an adjunctive agent. Market research confirms that, in patients with PD, the levodopa dose is adjusted in around 80% of patients, while only 20% have adjunct therapy as a first-line option. Adjusting the levodopa dose, either by increasing or fractionating the dose, or both, remains a valid, tried-and-tested option, although it has limitations. Joaquim Ferreira presented emerging evidence from a Phase II clinical trial, suggesting a potential benefit of adding opicapone 50 mg compared with 100 mg levodopa to treat patients with PD and end-of-dose fluctuations. This symposium aimed to present the effect of opicapone with relatively low total daily doses of levodopa; an option that may not have been traditionally considered by neurologists who are used to adjusting levodopa as a first-line response.
Motor Fluctuations in Parkinson’s Disease
Susan Fox
Fox emphasised that PD is one of the fastest-growing neurological disorders. Disability and deaths related to it have more than doubled from 1990–2015. Its prevalence is expected to grow exponentially by 2040, affecting approximately 12.9 million individuals worldwide.1
Since its introduction in 1967, levodopa has been the gold standard therapy for PD.2 However, long-term usage of high doses of levodopa may lead to complications, which are usually associated with advanced stages of PD, as the risk of developing MF and dyskinesia also increases with longer disease duration, but not with levodopa treatment duration.3,4 Yet, research shows that motor complications can also occur in the early phases of PD.
In a cross-sectional, questionnaire-based study in Europe, responses were collected from 817 patients with PD with an average disease duration of 3.3 years (mean Hoehn and Yahr score of 2.1).5 Results from this study indicated the presence of motor complications in 33% of patients, peak-dose dyskinesia in 15% of patients, and ‘OFF’ dystonia in 6% of patients.5 Presence of ON–OFF fluctuations were associated with poorer quality of life as measured by the EuroQoL 5-dimension (EQ-5D) questionnaire and Parkinson’s Disease Questionnaire-39 (PDQ-39).5 In another prospective longitudinal study in the UK, 734 patients with PD were followed for 10 years from diagnosis. Findings indicated the presence of motor complications in early PD and showed that higher levodopa dose, younger age, and baseline non-motor symptoms were associated with increased risk of such complications.6 Fox emphasised that a possible strategy to delay the development of MF in early PD and the progression of MF into the advanced disease stage would be to keep low doses of levodopa while adding adjunctive therapies, with the aim of achieving an extended duration of action and increased levodopa exposure through a continuous dopaminergic stimulation strategy.2
Dose Response to Levodopa Changes Over Time
Fox mentioned that the pharmacodynamic response to levodopa treatment changes over time. In early PD, administration of levodopa results in a linear dose–response (i.e., increased response with increased dose).7 However, as the disease progresses, higher doses of levodopa might be used to achieve a clinical response, which may result in peak-dose dyskinesia. Patients with advanced PD commonly experience the ’ON–OFF’ phenomenon and increasing the dose further may extend the duration of peak-dose dyskinesia.7 Fox highlighted that overcoming this issue remains a challenge for healthcare professionals treating patients with PD and that it might be avoided using the lowest possible levodopa doses.
Pathophysiology of Levodopa-Induced Complications
Fox believes the answer to why patients develop such levodopa-induced motor and non-motor complications remains complex. Decades of research indicate that the short duration of action of levodopa results in intermittent pulsatile stimulation of dopamine D1 receptors and multiple changes within the secondary messenger system, which ultimately leads to MF.8,9 Published evidence suggests that abnormal plasticity drives levodopa-associated motor complications at both pre- and post-synaptic levels.8,10 Firstly, as the disease progresses, there is a loss of pre-synaptic dopamine terminals in the nigrostriatal pathway and altered synaptic structure.10 The exogenously derived striatal dopamine is not stored well or cleared, resulting in large fluctuations in extracellular dopamine levels.8 Secondly, the peripheral metabolism of levodopa results in a short half-life due to various gastrointestinal factors, including dietary competition with protein in the gastrointestinal tract and across the blood–brain barrier; slowed gastric emptying due to disease, constipation, anticholinergics; and potentially Helicobacter pylori infection.11-13 Lastly, and most importantly, post-synaptic mechanisms within the striatum affect dopamine and non-dopaminergic neurotransmission. This is due to abnormal pulsatile stimulation of postsynaptic dopamine D1 receptors, leading to changes in the second messenger signalling pathways and altered neuron firing patterns.10 “Our research is focused on trying to prevent this intermittent pulsatile stimulation,” Fox emphasised, intending to prevent the development of MF.
In concluding her talk, Fox highlighted the need for alternative therapeutic options to provide continuous dopaminergic stimulation while maximising the benefit of levodopa and presented a clinical case of a patient with recently diagnosed MF. Fox asked the audience how they would manage the patient’s symptoms among different options, such as adding a catechol-O-methyl transferase (COMT) inhibitor, reducing the time interval between levodopa doses, starting a monoamine oxidase-B inhibitor, stopping the dopamine agonist they were receiving, and increasing the levodopa dose. Fox then presented a rationale for choosing or not choosing each of the options above.
Newly Emergent Motor Fluctuations: What We Think We Know
Angelo Antonini
Antonini’s lecture explored and discussed the validity of the most common approaches routinely used to treat MF. An online market research survey (unpublished data, sponsored by Bial) of healthcare professionals (n=409) with extensive experience in treating patients recently diagnosed with MF (n=1,636) was conducted across six European countries. In 81% of patients (n=656), immediate change in levodopa dose was the preferred approach for managing MF versus adding an adjunct therapy (21% of patients [n=173]). However, more than twice as many movement disorder specialists (31%) versus general neurologists (15%) preferred an adjunctive treatment as the first-line option, suggesting that clinical experience in PD management is important (unpublished data, Bial).
The Goal of Optimising Levodopa Pharmacokinetics
As a prodrug, levodopa is converted to dopamine. When it crosses the blood–brain barrier, levodopa is decarboxylated to dopamine, released from the synaptic cleft, and stimulates the dopaminergic receptors, compensating for the depleted supply of endogenous dopamine in PD. Antonini briefly reviewed the concept of continuous dopamine delivery, which would mimic the normal physiological state of the brain. In this state, dopaminergic neurons fire tonically, providing an almost steady dopamine flow to the dopamine receptors. In PD, dopamine release becomes phasic and spaced out due to the gradual loss of dopaminergic neurons.9,14 Disease progression with 70%–80% of neuron loss affects the pre-synaptic storage capacity, resulting in phasic firing, leading to peak and trough fluctuations in striatal dopamine levels. This is primarily due to a progressive decrease in the ability of the nigrostriatal neurons to synthesise and store dopamine formed from exogenous levodopa, causing a reduction in the long duration and an increase in the magnitude of the short duration response.14,15 Eventually, patients are deemed ‘fluctuators’ when the benefit of levodopa is dependent on the magnitude of the short duration of levodopa response, explained Antonini.15
Levodopa treatment in the early stages of PD may restore normal striatal dynamics and tonic release of dopamine from presynaptic terminals; however, the short half-life of levodopa also leads to its pulsatile delivery to the brain and the development of motor complications.11 Continuous dopaminergic stimulation more closely resembles the regular physiological phasic dopamine release and may better facilitate movement control.9,14
Understanding levodopa pharmacokinetics could explain the reasons behind optimal and effective treatment options. An increase in levodopa exposure (via increases of the area under the curve) increases the relative bioavailability of levodopa to be converted into dopamine.16 Through a continuous delivery approach, one can increase levodopa troughs (i.e., increase minimum observed plasma concentration), which may lead to a decrease in OFF-time and time-to-ON (Figure 1).16,17 By controlling the levodopa plasma peaks (control maximum observed plasma concentration), one can reduce the risks of developing dopaminergic adverse events (AE).16,17 Therefore, pharmacokinetic optimisation of levodopa can increase its bioavailability while minimising motor complications and AEs.16,17
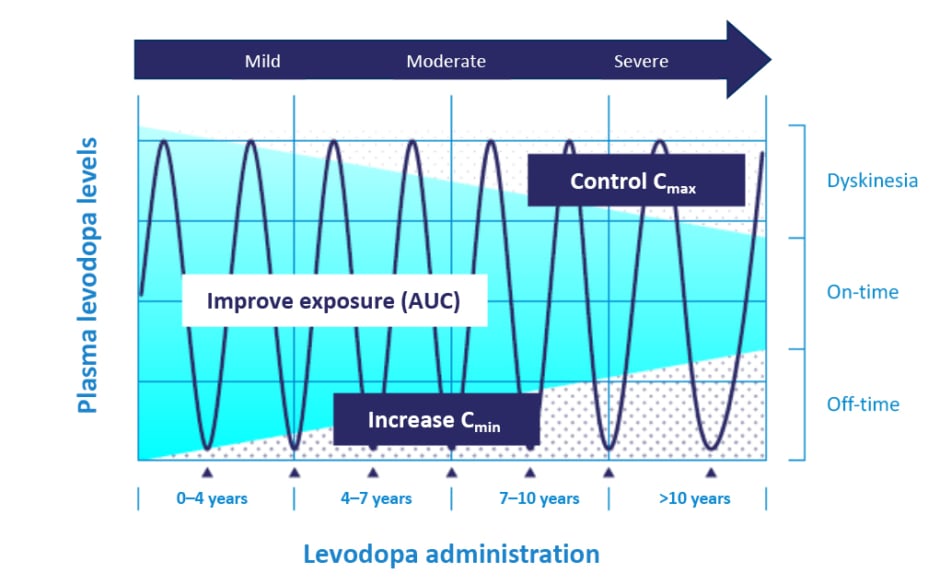
Figure 1: Pattern of motor response towards levodopa changes during Parkinson’s disease progression.
Mild refers to early onset of PD for 0–4 years, moderate refers to 4–10 years, and severe refers to more than 10 years of PD.
Adapted from Cenci.17
AUC: area under the curve; Cmax: maximum observed plasma concentration; Cmin: minimum observed plasma concentration; PD: Parkinson’s disease.
Levodopa Dose Increase Versus Dose Fractionation
Levodopa dose adjustment (dose increase or fractionation) is still the most common approach for patients with early MF (unpublished data, Bial). However, Antonini scrutinised this approach and highlighted that increasing the amount of each dose of levodopa does not eliminate plasma level troughs, and it may lead to increased pulsatility and a greater incidence of dyskinesia.18 Similarly, levodopa dose fractionation is ineffective at reducing troughs in plasma levels and is often associated with intermittent re-emergence of symptoms due to suboptimal levodopa exposure.18 Results from the ELLDOPA trial19 suggested that levodopa is the most effective drug to manage PD. Patients taking levodopa 150 mg/day (n=92) had a significantly lower risk of dyskinesia versus those taking levodopa 600 mg/day (n=91; 3.3% versus 16.5% at 42 weeks; p<0.001).19 Similarly, a sub-analysis of the STRIDE-PD study20 demonstrated a significant levodopa dose-dependent increase in wearing-off and dyskinesia risk (p<0.001 for both) and supported Cilia et al.’s3 observations that motor complications and dyskinesia are not associated with the duration of levodopa therapy but rather with longer disease duration and higher levodopa dose.3,21 Antonini concluded the session by stating that levodopa remains the gold standard treatment.2 Adjustment of its dose remains a common approach to treating recently diagnosed MF, but therapeutic strategies to limit the increases of levodopa doses should be preferred to optimise its pharmacokinetics.21
Newly Emergent Motor Fluctuations: What We Thought We Knew
Joaquim Ferreira
Ferreira opened his presentation by discussing that one alternative approach to optimise levodopa pharmacokinetics would be using enzyme inhibitors such as dopa-decarboxylase inhibitors (carbidopa and benserazide), COMT inhibitors (tolcapone, entacapone, and opicapone), and monoamine oxidase-B inhibitors (selegiline, rasagiline, and safinamide).22 Research indicates that dual inhibition of dopa-decarboxylase and COMT may cause a 30–50% reduction in plasma variability of levodopa.22,23
Opicapone as a Levodopa Optimising Agent
Ferreira emphasised that peripheral levodopa would need to be optimised to compensate for the drop in dopamine levels in the brain in patients with PD.22 Ferreira discussed the utilisation of opicapone (50 mg) as an adjuvant to optimise levodopa levels in the brain as an alternative option to directly adjusting the levodopa dose.24,25
Ferreira asked the audience about their expected range of change in levodopa exposure after adding opicapone 50 mg and decreasing the daily levodopa dose by 100 mg (from 500 mg). Overall, 44% of responding attendees believed that levodopa exposure would increase by 10–20%. To explore this question further, Ferreira described results from a Phase II, open-label, modified crossover, exploratory trial (EudraCT number: 2020-003139-12) to study the effect of opicapone 50 mg on levodopa pharmacokinetics with different levodopa/carbidopa regimens in patients experiencing end-of-dose MF.25 The effect of opicapone 50 mg on levodopa pharmacokinetics (total levodopa daily dose 400 mg) compared with baseline (total of 500 mg levodopa [without opicapone]) was the primary endpoint. Secondary endpoints included tolerability, ON- and OFF-time (measured using 12-hours ON/OFF diaries), and Patient Global Impression of Change (PGI-C).25 Patients received levodopa/carbidopa 400/100 mg, either in four intakes (levodopa 100 mg at 4 hour intervals) plus opicapone 50 mg once daily (n=12), or in five intakes (levodopa 100/50/100/50/100 mg at 3 hour intervals) plus opicapone 50 mg once daily (n=12), for up to 14±2 days, and were compared with baseline levodopa/carbidopa 500/125 mg in a five-intake regimen (at 3 hour intervals).25 Results indicated improvements in the pharmacokinetic profile of levodopa upon adding opicapone 50 mg despite a decrease of 100 mg levodopa in patients with PD and end-of-dose MF (Figure 2), with subsequent benefits in the motor response (Figure 3).25
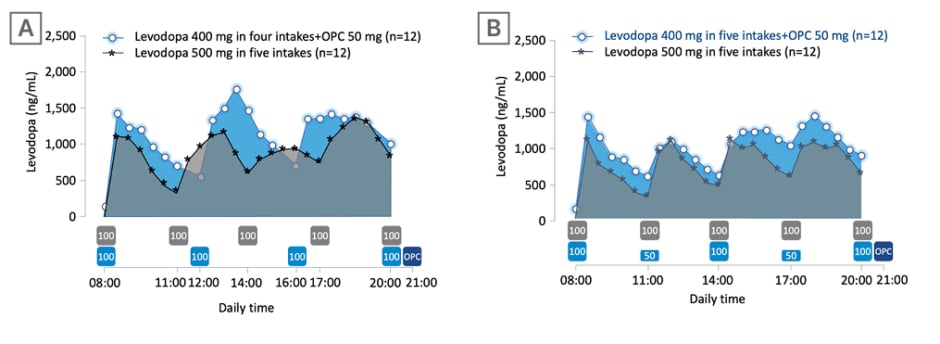
Figure 2: Response to levodopa therapy in relation to levodopa pharmacokinetics.
Mean levodopa plasma profile versus time following 2-week five-intake (every 3 hours) daily oral administrations of levodopa/carbidopa 500/125 mg, compared with 2-week four-intake (every 4 hours) daily oral administrations of levodopa/carbidopa 400/100 mg plus once-daily opicapone 50 mg (A), or compared with 2-week, five-intake (every 3 hours) daily oral administrations of levodopa/carbidopa 400/100 mg plus once-daily opicapone 50 mg (B). The boxes under the graphs indicate when levodopa (grey box for baseline regimen and light blue for test regimens; dose in mg) and opicapone (dark blue) were taken.
Adapted from Ferreira et al.25
OPC: opicapone.
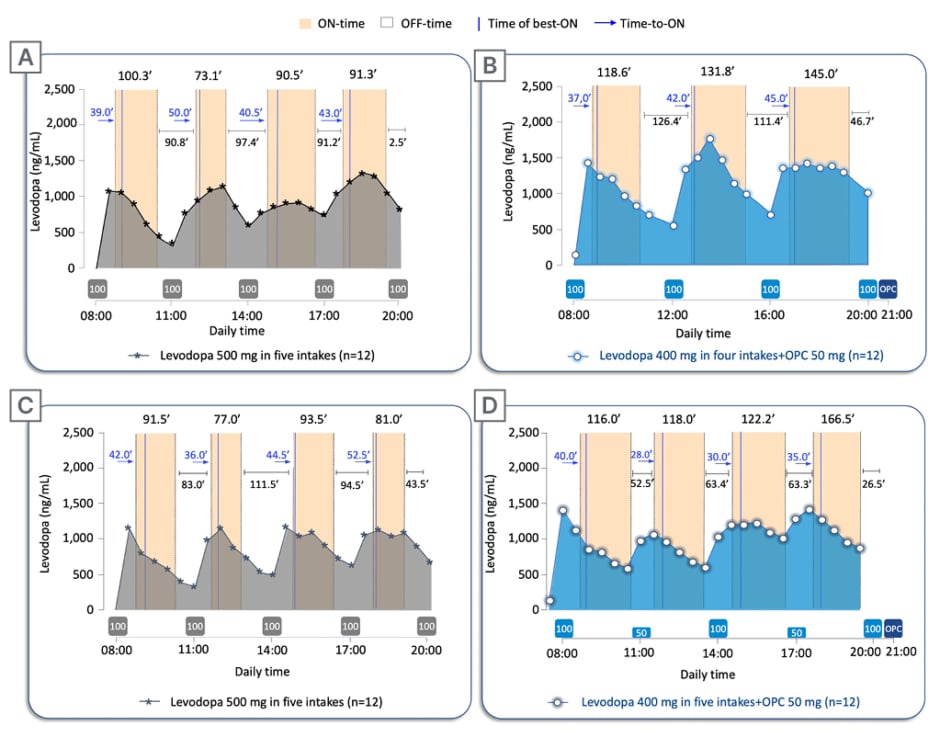
Figure 3: Twelve-hour ON-/OFF-time data on pharmacokinetic assessment days.
Twelve-hour ON-/OFF-time data reported on pharmacokinetic assessment days superimposed to the mean levodopa plasma profile versus time following: A) 2-week five-intake (every 3 hours) daily oral administrations of levodopa/carbidopa 500/125 mg without opicapone, compared with B) 2-week four-intake (every 4 hours) daily oral administrations of levodopa/carbidopa 400/100 mg plus once-daily opicapone 50 mg; and C) 2-week five-intake (every 3 hours) daily oral administrations of levodopa/carbidopa 500/125 mg without opicapone, compared with D) 2-week five-intake (every 3 hours) daily oral administrations of levodopa/carbidopa 400/100 mg plus once-daily opicapone 50 mg. The boxes under the graphs indicate when levodopa (grey box for baseline regimen and light blue for test regimens; dose in mg) and opicapone (dark blue) were taken. Time of best-ON and Time-to-ON are reported in minutes.
OPC: opicapone.
Furthermore, patients experienced an improvement in the PGI-C upon adding opicapone to levodopa treatment in both treatment arms.25 In the four-intake levodopa/carbidopa 400/100 mg plus opicapone 50 mg regimen, 66.7% of patients (n=12) reported an improvement on the PGI-C (very much/much/minimal improvement) with 33.4% experiencing ‘much/very much improvement’.25 Similarly, with the five-intake levodopa/carbidopa 400/100 mg plus opicapone 50 mg regimen, 91.7% reported improvement on the PGI-C (very much/much/minimal improvement), with 41.7% experiencing ‘much improvement’. ‘Much’ and ‘very much’ worsening was not reported in either of the treatment arms and ‘minimally worse’ was reported in 8.3% patients in the four-intake arm only. Overall, addition of opicapone 50 mg to levodopa/carbidopa treatment regimen was well-tolerated.25 Only two AEs were reported during the study (blood glucose and gamma-glutamyl transferase increased), both in the four-intakes arm. Despite the increased levodopa exposure with opicapone versus baseline, no patients reported dyskinesia as an AE.25
To further clarify the role of opicapone in the optimisation of levodopa, the ADOPTION trial26 is an ongoing Phase IV, randomised, open-label, exploratory trial being conducted in 25 European sites. It aims to explore the potential of opicapone 50 mg to optimise levodopa/dopa-decarboxylase inhibitors as a first-line approach to treat wearing-off (stable treatment plus the addition of opicapone 50 mg versus an additional 100 mg levodopa/carbidopa) in approximately 100 patients with signs of wearing-off for less than 2 years, and treated with 3–4 daily oral levodopa doses (up to 600 mg) in a 4-week period.26 These inclusion criteria are a crucial aspect of the ADOPTION trial, as patients have less severe motor fluctuations (total daily OFF-time ≤5 hours) than those observed in Phase III trials, which led to the current indication of opicapone. The primary endpoint is the change from baseline in ‘OFF’ time at week 4. Secondary endpoints include tolerability, functional motor, and non-motor assessments through questionnaires, Hauser’s home diary, and PGI-C.26 Ferreira closed the session by describing that the ongoing studies and specifically the ADOPTION study, will help assess the effect of opicapone across the whole spectrum of MF,26 and not only in mid-to-late stages of PD, as traditionally accepted for COMT inhibitors so far.
Opicapone prescribing information can be found here.
For UK HCPs only, opicapone prescribing information and adverse event reporting can be found here.
ON/NOV22/G/202
Date of preparation: November 2022








