Meeting Summary
More than half of survivors of stroke experience some degree of motor impairment, and spasticity can develop within days of the initial event. Patients with post-stroke spasticity (PSS) report a lower quality of life than those without spasticity, and they require regular long-term follow-up and monitoring within the healthcare system.
This symposium supported a non-promotional discussion regarding the prevalence, burden, consequences, and need for identification of PSS. The benefits of PSS identification within 3 months of stroke were discussed by a panel of key opinion leaders, including Ted Wein, Neurologist and Assistant Professor of Neurology and Neurosurgery at McGill University, Montréal, Quebec, Canada; Ganesh Bavikatte, Consultant and Clinical Lead in rehabilitation medicine at the Walton Centre, Liverpool, and Honorary Senior Clinical Lecturer at the University of Liverpool, UK; and Sean Savitz, Professor of Neurology and Physical Medicine and Rehabilitation, Frank M. Yatsu MD Chair in Neurology, and Director of the Institute for Stroke and Cerebrovascular Diseases, University of Texas Health Science Center at Houston (UTHealth), Texas, US. These key opinion leaders explained that early prediction of PSS could be improved by increased awareness of the associated risk factors and tools, such as the Post-Stroke Checklist (PSC), the Spasticity Screening Tool, and the PSS Referral Tool. Finally, potential barriers to the early identification of PSS were presented, alongside strategies to overcome these barriers.
Early Identification of Post-stroke Spasticity: What Could it Mean for Patients?
Many patients experience impairment following a stroke, including motor or cognitive impairment, urinary incontinence, dysphagia, language impairment, and psychological disturbances.1 Motor impairments are the most common post-stroke complication, experienced by 50–83% of patients.1
Spasticity is a common consequence of stroke,2,3 and is characterised as disordered sensorimotor control resulting from an upper motor neuron lesion, presenting as intermittent or sustained involuntary activation of muscles.4 Spasticity may lead to hypertonicity, disability, reduced function, and associated pain.5
Spasticity primarily affects the elbows, wrists, and ankles,6 though it has also been reported in other locations, such as the finger and toe flexors, and shoulder and thigh adductors (Figure 1).2,7 Around 63% of patients with PSS experience both upper and lower limb spasticity.8
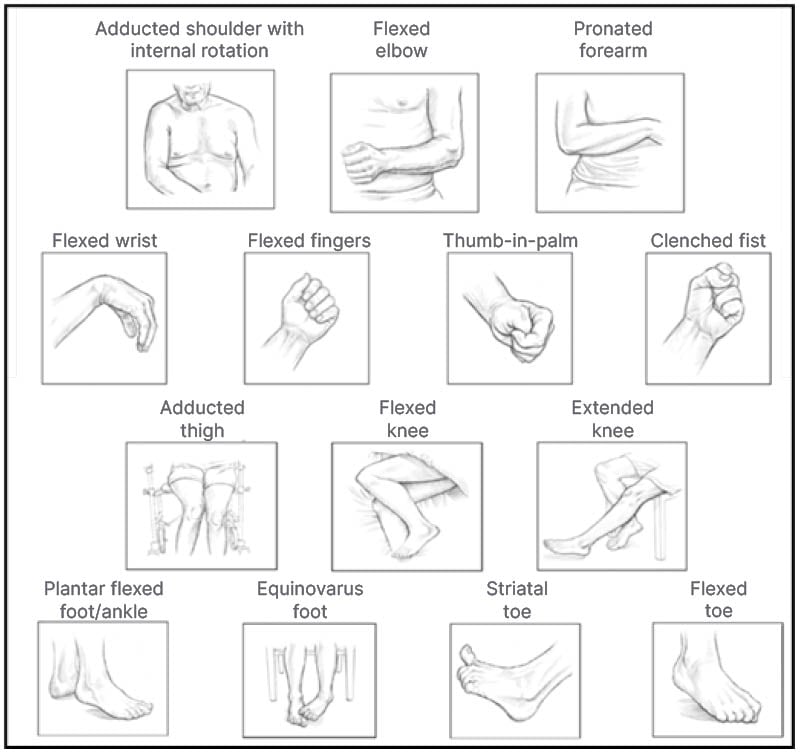
Figure 1: Common postures of spasticity in upper and lower limbs.
Images reproduced from Simpson DM et al.9 and Esquenazi A et al.,10 open-access articles under the terms of the Creative Commons Attribution 4.0 International Licence
(https://creativecommons.org/licenses/by/4.0/).
Approximately 100 million people are living with stroke worldwide, of whom 22% are individuals between 15–49 years of age.11 The prevalence of PSS increases over the first 6 months post-stroke,12 and estimates anticipate a 27% increase in the demand for post-stroke rehabilitation in the European Union (EU) over the next 3 decades.13
It was emphasised that spasticity can have a broad impact on a patient’s functional recovery and general wellbeing, potentially leading to contractures, pain, insomnia, depression, pressure ulcers, compromised mobility, increased dependency on others, difficulty maintaining sexual relations, poor self-esteem, and difficulty maintaining hygiene.3,14,15
Mobility limitations and limb impairments may result in serious physical and emotional burdens for patients and caregivers. For example, a lack of motor control can result in frequent falling,3 caregivers may need to provide postural support management. Assistance with hygiene and dressing is frequently required.3,16 In addition, patients’ and caregivers’ ability to work may be affected.17 A prospective study reported that 3 months after ischaemic stroke, patients reporting spasticity (n=54) had significantly lower health-related quality of life scores compared with patients not reporting spasticity (n=274), indicating that patients with PSS may experience psychological and emotional issues.18 Further studies have demonstrated that significantly more patients with PSS (n=18) experienced pain at a median of 16 weeks post-stroke, compared to patients without PSS (n=65; 72.0% versus 1.5%; p<0.001),6 and between 35–48% of patients with PSS (n=273) reported that pain resulted in moderate-to-extreme work interference.12
The incidence of spasticity, particularly severe/disabling spasticity, generally increases over time following the initial stroke.19 A systematic review and meta-analysis of 23 studies indicated that in the first month following a stroke, 4–46% of patients experienced spasticity, and 2–3% experienced severe/disabling spasticity. At 3–6 months following a stroke, these figures increased to 7–63% and 8–16% of patients, respectively.19
Several studies have shown that PSS can develop in a matter of days after a stroke, with one study reporting that spasticity was present in 25% of patients at Day 3;20 another that spasticity was present in 21% of patients at an average of 5.4 days following stroke;7 and a third that 25% of patients showed an increase in muscle tone in at least one joint in the first 14 days after stroke.6
If PSS is left untreated, a vicious cycle can develop, in which unopposed contraction causes abnormal limb posture, leading to soft tissue shortening, further biomechanical changes in contracted muscles, and prevention of muscle lengthening, thus perpetuating further tonicity.3
Management options available for PSS include pharmacological treatment (e.g., oral treatments, injectables such as botulinum toxin type A [BoNT/A]), physical rehabilitation (e.g., physical and occupational therapy with a multidisciplinary team),3 and surgical approaches (e.g., destructive and orthopaedic procedures).21
BoNT/A is a long-acting, focally-administered neuromodulator that is recommended for the treatment of PSS.22 BoNT/A therapy should be considered when spasticity interferes with the goals of patients, caregivers, or healthcare teams, regardless of the time since diagnosis,23 and studies have indicated that early intervention with BoNT/A within 3 months after onset, rather than later, may deliver a greater reduction in muscle tone,24 and may minimise the risk of developing contractures.25
A meta-analysis of six studies reporting the effects of BoNT/A treatment less than 3 months after stroke onset indicated that early treatment with BoNT/A resulted in a significant treatment effect (p=0.0002), in terms of reduction in hypertonicity compared to controls.5 Furthermore, a multicentre, longitudinal cohort study indicated that BoNT/A treatment for PSS initiated within 3 months of stroke onset may result in greater reductions in muscle tone at Months 1 and 3 of follow-up, compared with treatment initiated later.24 This study was conducted in patients who have had a stroke with PSS, who received BoNT/A for the first time, according to routine practice, within 12 months of stroke onset (n=83). Results showed that the odds of increased muscle tone in patients treated with BoNT/A more than 90 days after stroke onset versus patients treated within 90 days were 2.1-fold greater at Month 1 of follow-up (p=0.044), 2.9-fold greater at Month 3 of follow-up (p=0.002), and 1.1-fold greater at Month 6 of follow-up (p=0.747).24 In addition, a randomised controlled trial indicated that patients who developed spasticity within 6 weeks of a first stroke (n=93), who received BoNT/A injections versus placebo, were shown to have a significantly greater passive range of motion in the wrist at 6 weeks post-treatment (75.6 degrees versus 63.8 degrees, respectively [p=0.004]).25
It was concluded that spasticity is a common consequence of stroke,2 and patients with PSS report significantly lower health-related quality of life scores than patients with stroke without spasticity.18 The incidence of spasticity increases over time, potentially reaching up to 46% of the stroke population within 1 month since stroke,19 and early BoNT/A treatment (<3 months after stroke) may result in improved outcomes for patients with PSS.5,24,25
What Tools Can Be Used to Identify and Assess Post-stroke Spasticity Early?
Early identification and prompt treatment of PSS is important because spasticity may evolve less than 3 months after stroke,5 and early treatment with BoNT/A can improve muscle tone,24 spasticity-related pain,5 and passive range of motion.25
Recognition of the risk factors associated with PSS can enable the early prediction of PSS in a patient who has had a stroke.12 In the acute phase (the first week) following stroke, risk factors include a low Barthel Index score and EQ-5D,6 hemihypaesthesia,8 severe paresis,6,8,26,27 and increased muscle tone.6 In the post-acute phase, risk factors include a low Barthel Index score,6,8 paresis (particularly if limited to one side of the body),6,28 extensive stroke lesions on CT28 and MRI,29 a National Institutes of Health Stroke Scale (NIHSS) score indicative of severe stroke,31 and a low Motricity Index.30
Alterations in the modulating influence of descending fibre tracts on local spinal networks are widely accepted as relevant pathophysiological changes in patients with PSS. However, not all ischaemic brain lesions result in PSS.29 Several studies have indicated that consideration of the site and size of stroke lesions measured on MRI may help to predict PSS development.29,31,32 In one retrospective analysis of MRI data from patients with stroke with (n=39) and without (n=59) PSS, lesions over 3 cm3 in diameter, located within the middle cerebral artery territory with involvement of the pyramidal tract and/or internal capsule, were found to be predictive of PSS (odds ratio: 28.48).29 In another retrospective study, voxel-based lesion symptom mapping with MRI images from patients who have had a stroke (n=45) found that the involvement of the striatum and white matter tracts influenced the development of spasticity in the upper and lower limbs.32
Several tools are available to support the early identification of PSS, including the Moving Beyond Stroke initiative, to empower patients and caregivers to speak to their healthcare practitioners (HCP).33 The PSC is designed to be completed by patients, and links patient-reported problem areas, including spasticity, pain, mobility, mood, and activities of daily living to a specific referral, such as a primary care physician, rehabilitation/spasticity specialist, or speech language therapist.34 The Spasticity Screening Tool is a brief, patient-reported questionnaire designed to identify muscle stiffness, tightness, or spasms experienced over the past month; it can be used in general practice and specialist settings to enhance the identification and appropriate referral of patients with spasticity.35
The PSS Referral Tool is an aid to identify and triage patients who are at risk of developing PSS, and to provide guidance on patient management.36,37 The PSS Referral Tool was developed from consensus recommendations for spasticity management and rehabilitation in clinical practice, and was created by a panel of expert specialists. The expert consensus panel agreed that identification of spasticity and its risk factors, and a lack of subsequent referral to rehabilitation or specialist spasticity services, is a key unmet need. The PSS Referral Tool aids the categorisation of patients who have had a stroke into three levels of risk: red (urgent referral), amber (routine referral), and green (periodic monitoring; Figure 2).37
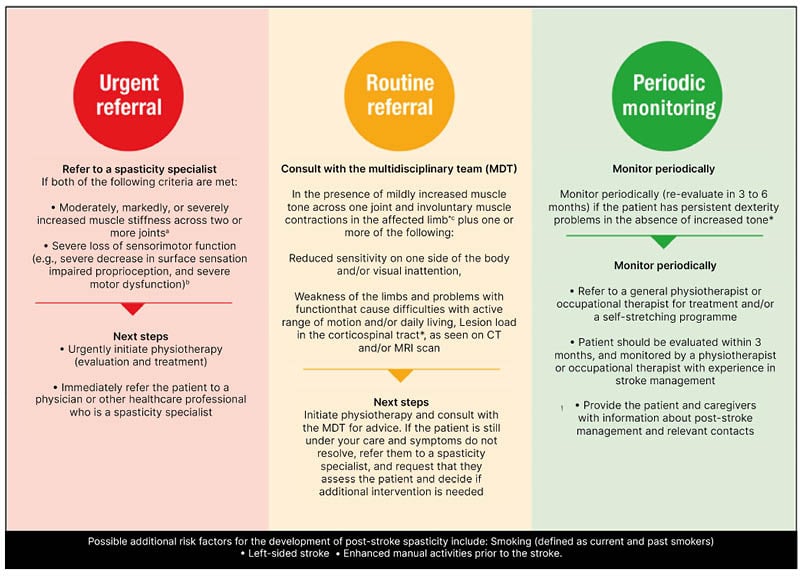
Figure 2: Post-stroke spasticity referral tool.
A) Mildly increased muscle stiffness is a Modified Ashworth Scale (MAS) 1 or +1, while moderately is MAS 2, markedly is MAS 3, and severe is MAS 4. B) Measured using the FMUE scale. C) Muscle contractions may occur due to spasms, disturbed reciprocal inhibition, or spastic dystonia, and should be differentiated from contractures. D) Visual inattention includes hemianopsia, scotoma, or visual neglect. E) Can be measured with the Barthel Index (low score) and EQ-5D (low score).
Figure adapted from Bavikatte G et al.,38 an open-access article under the terms of the Creative Commons Attribution-NonCommercial 4.0 License (https://creativecommons.org/licenses/by-nc/4.0/).
FMUE: Fugl-Meyer Upper Extremity; MDT: multidisciplinary team.
The tool is recommended for use by clinicians and physiotherapists when evaluating patients who have had a stroke, both in primary and secondary care. Ideally it should be used within 12 weeks of the stroke event, and it should also be used at regular follow-up visits.36,37 It was explained that the PSS Referral Tool could be used to aid patient triage; to highlight early identification of PSS in clinics and/or institutions that do not currently consider it a main focus; to define referral strategies based on severity of spasticity; and to increase awareness of PSS symptoms, and the different referral options.
A global inter-rater reliability study has recently been completed to validate the PSS Referral Tool, to ensure that the tool possesses sufficient sensitivity and specificity for meaningful use by clinicians, regardless of experience and region of practice.38 In Part A, investigators videoed the clinical assessment of 30 patients with varying degrees of spasticity using a semi-structured script based on the PSS Referral Tool. In Part B, the videos were classified by an expert global panel using the PSS Referral Tool, and in Part C, inter-rater reliability was performed virtually on 15 of the videos (five for each referral category) by stroke rehabilitation clinicians from six global regions. It was noted that data from this study is currently being analysed, and that it is hoped that results will lead to validation of the tool within the target audience, 6-monthly reviews post-stroke becoming standard practice, and long-term improvements in disease awareness of PSS amongst the relevant HCPs.
A mobile app has also been developed to host the PSS Referral Tool, and to support HCPs in the early identification of PSS and appropriate referral. The PSS: Early ID™ app (AbbVie, North Chicago, Illinois, USA) aims to provide the healthcare community with access to on-demand educational content, which may help with awareness and early identification of PSS, both through the PSS Referral Tool and through the ‘My Clinic’ section of the app, which includes hypothetical patient case studies. The app also has the potential to understand how confident HCPs are at identifying PSS (Bavikatte, personal communication). The target audience for the app includes neurorehabilitation consultants, physical medicine and rehabilitation physicians, primary care physicians, neurologists, stroke physicians, acute physicians, physical therapists, nurses, and occupational therapists.
In conclusion, PSS may be predicted and triaged at an early stage by looking out for certain risk factors in patients, and there are tools available to support the early identification and prompt management of PSS, which are self-explanatory, and easy to use. New tools are being developed to support the classification of PSS risk for referral purposes, and to increase awareness of PSS among HCPs. The panel emphasised the importance of actively involving patients in their care and wellbeing, and underscored the value of tools like the PSC, to support communities which are underserved, or do not have good access to medical care.
What Can We Do to Help Drive the Early Identification of Post-stroke Spasticity in the Acute Stroke Setting?
Rehabilitation and recovery is a key aspect of the continuum of stroke care,39 and often includes passive stretching and/or repetitive task practice.3 However, there is limited data available to support guidelines for this stage of care,3,39 and it was stressed that spasticity detection is often not addressed in the acute care setting for patients who have had a stroke.
There are several potential barriers that may be responsible for this situation (Figure 3):
- Lack of understanding: HCPs, patients, or caregivers may not recognise the symptoms of spasticity, and patients may be unaware of the possible impact of spasticity and/or more concerned by other symptoms post-stroke.40
- Transition from acute care: Acute-care therapists may not assess spasticity, and there is a focus on early supported discharge41 (Savitz, personal communication).
- Logistical challenges: There may be delayed access to specialist referral from acute care, and patients may find it difficult to access rehabilitation centres or physiotherapy.40
- Attitudes towards spasticity: At the acute care level, there may be a lack of communication among the multidisciplinary team, and in the chronic phase of stroke, spasticity symptoms may not be considered by HCPs to be the most important complication of stroke.40
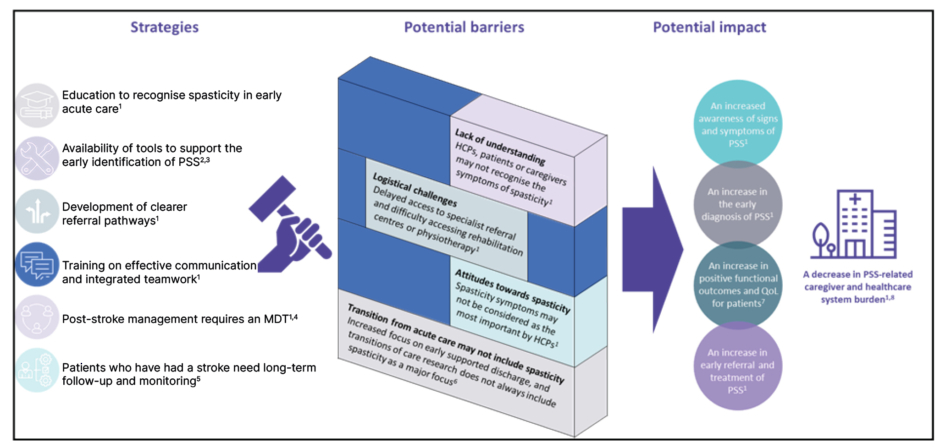
Figure 3: Strategies to help drive the early identification of post-stroke spasticity in the acute
stroke setting.
HCP: healthcare professional; MDT: multidisciplinary team; PSS: post-stroke spasticity; QoL: quality of life.
Strategies that may help to support the early identification of PSS in the acute care setting include the education of HCPs to recognise spasticity in early acute care; the development of clearer referral pathways; training on effective communication and integrated teamwork; 40 and increased availability of tools to support the early identification of PSS (Figure 3).34,35
Following a stroke, patients can experience a variety of symptoms which may affect their life, including pain and spasticity; problems with cognition, communication, mood, and mobility; incontinence; and activities of daily living.42 Secondary stroke prevention is also important.43 Because of the wide range of potential post-stroke symptoms and considerations, it was explained that a transdisciplinary approach is needed in the post-stroke setting. This may include rehabilitation nurses, stroke neurologists, speech and language therapists, social workers, physical therapists, vocational counsellors, occupational therapists, psychologists, physical medicine and rehabilitation specialists, and the patient and their family.3,40,43 It was emphasised that concerted effort by a multidisciplinary team is necessary to help ensure optimal post-stroke patient care (Figure 3).40,43
The long-term follow-up and monitoring required by patients after a stroke is being implemented through guidelines by healthcare providers and stroke organisations.39,41,44 For example, the World Stroke Organization (WSO) recommends that stroke rehabilitation should begin in the sub-acute phase,44 the American Stroke Association recommends that the provision of comprehensive rehabilitation programmes should be a priority,41 and the Canadian Best Practice Recommendations for Acute Stroke Management state that patients with stroke and neurological impairments that could impact daily functioning should be referred to an appropriate rehabilitation specialist.39
Working towards an early diagnosis of PSS could lead to increased awareness of signs and symptoms of PSS, an increase in early referral and treatment of PSS, and an increase in positive functional outcomes and quality of life for patients.37,40 Together, these changes have the potential to decrease the PSS-related burden on caregivers and the healthcare system (Figure 3).13,40
Finally, the panel asked that HCPs make the following pledge to help to overcome the barriers to early identification of PSS in the acute stroke setting:
I am committed to becoming better educated in recognising the signs and symptoms of PSS; if I do not have the expertise to manage spasticity, I will refer my patients to specialists with appropriate expertise; and I will aim to refer appropriate patients with PSS to clinical studies in the future.
Summary and Closing Remarks
The prevalence of PSS increases in the first 6 months following stroke, and that BoNT/A treatment within 3 months of stroke may result in improved patient outcomes, including the prevention of secondary complications, such as contractures. It was clarified that certain risk factors can help to predict PSS at an early stage, and that there are tools available to support the early identification of PSS, such as the PSC, the Spasticity Screening Tool, and the PSS Referral Tool. Several key actions can be taken in the acute stroke setting to help support early identification of PSS, including improved education, training, and communication, as well as clear referral pathways.







