Meeting Summary
This symposium took place during the International Society on Thrombosis and Haemostasis (ISTH) Congress, 2022. Ajay Kakkar, Thrombosis Research Institute, London, UK, opened the symposium by highlighting the extent to which patients with cancer are affected by thrombotic disease. Subsequently, Mari Thomas, University College London, UK, described the current treatment options for patients with cancer-associated thrombosis (CAT). Thomas highlighted the current unmet needs faced by these patients, with the risk of bleeding continuing to be the main issue affecting anticoagulation treatment. Paula Bolton-Maggs, University of Manchester, UK, and UK Haemovigilance Scheme, Manchester, UK, gave an overview of Factor XI (FXI) inhibition and outlined why this factor is a potential attractive anticoagulation drug target. Multiple therapies targeting FXI are currently in development, with seven clinical trials published to date.1-7 Although there are some similarities between these agents, there are also many differences in terms of their administration route and frequency, the timing of onset of action, and methods of metabolism and excretion. Giancarlo Agnelli, University of Perugia, Italy, and Istituti Clinici Scientifici (ICS) Maugeri, Pavia-Milan, Italy, then described the importance of conducting clinical trials that are designed to address the unmet needs of patients with CAT. In particular, he highlighted the ongoing Phase III ASTER and MAGNOLIA studies that are aiming to demonstrate that the FXI inhibitor abelacimab is superior to standard of care treatments in terms of bleeding rates for patients with CAT, including those with gastrointestinal (GI) or genitourinary (GU) tumours. Concluding remarks were given by Gary Raskob, University of Oklahoma Health Sciences Center, Oklahoma City, USA, who re-emphasised the unmet needs in the treatment of CAT, and the promise that FXI inhibitors hold for the future.
Opening Remarks
Ajay Kakkar
Kakkar opened the symposium by highlighting the burden of thrombotic disease in patients with cancer with a discussion of data from the Global Anticoagulant Registry in the FIELD – Venous Thromboembolism (GARFIELD-VTE) registry.8 Patients with active cancer and a confirmed diagnosis of venous thromboembolism (VTE) had a two-fold increased risk of recurrent VTE, a five-fold increased risk of a major bleed, a four-fold increased risk of a stroke or a transient ischaemic attack, and a 15-fold increased risk of all-cause mortality, compared with patients with a VTE but without cancer.8 An analysis of treatment patterns in the same study demonstrated the adoption of direct oral anticoagulants (DOAC), both in the short-term and long-term in patients with a VTE (Figure 1). However, it also revealed that over time, a large number of patients discontinued anticoagulation, with this often occurring quite early in the natural history of their thrombosis; for example, in the GARFIELD-VTE registry almost one-third of patients (30.4%) discontinued anticoagulation within 9 months of their original VTE.8 Undertreatment with anticoagulants has also been reported in the European PREFER in VTE Registry, where low use was reported in higher-risk patients.9 There are likely to be many reasons underlining this undertreatment; however, fear and risk of bleeding continue to be a major deterrent to optimal anticoagulation, even in the era of DOACs.
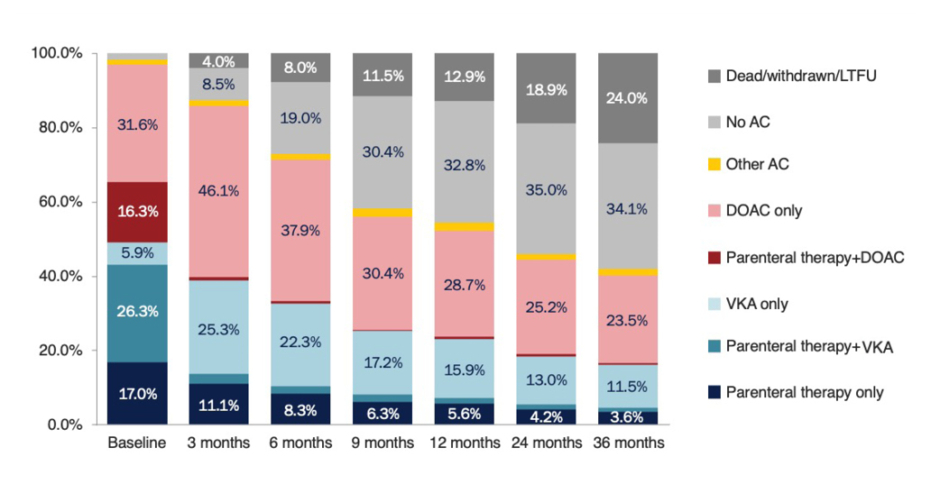
Figure 1: Patterns of anticoagulation prescription over time in patients with a venous thromboemolism enrolled in the GARFIELD-VTE registry.8
AC: anticoagulant; DOAC: direct oral anticoagulant; LTFU: long-term follow-up; VKA: vitamin K antagonist.
Kakkar concluded the introduction by highlighting the challenges faced in the management of high-risk patient populations, both in terms of addressing the pathophysiology underlying the thrombosis risk and implementing interventions that lead to favourable clinical outcomes. Ensuring that patients with cancer who already have a high burden of treatment can adhere to anticoagulation therapies over time is also vitally important.
Cancer-Associated Thrombosis: Burden of Disease and Current Treatment Options
Mari Thomas
Thomas began her presentation by describing the dramatic increase in the incidence of VTE in patients with cancer during the past 20 years.10 As patients with cancer are living longer, the incidence of CAT has increased markedly,10 where the main driver of this increase is the improvements in cancer treatments. However, the increased thrombotic risk associated with some of these anticancer therapies also needs to be taken into account. Thomas also emphasised the considerable burden associated with CAT, as illustrated by a study reported in 2007 by Khorana et al.,11 which reported that thromboembolism was the second leading cause of death in patients with cancer who were receiving outpatient chemotherapy.11 Although CAT is less common in early-stage cancer since these patients have a better prognosis, they may have more to gain from anticoagulant treatment.12 However, anticoagulation in patients with cancer can be more challenging than in patients without cancer, as highlighted in a study by Prandoni et al.,13 which evaluated outcomes for patients with a clinically suspected deep vein thrombosis (DVT) who received initial treatment with unfractionated heparin or low molecular weight heparin (LMWH) followed by a vitamin K antagonist (VKA) for at least 3 months.13 In this study, the 12-month cumulative incidence of a recurrent VTE was 20.7% in patients with concomitant cancer, compared with only 6.8% in patients without cancer.13 The 12-month cumulative incidence of major bleeding was also considerably higher in patients with versus without cancer (12.4% versus 4.9%).13 The outcomes for patients with CAT have improved; the introduction of DOACs has reduced the risk of recurrent VTE for patients with CAT,14 but the high risk of bleeding remains a key unmet need, particularly for patients with GI or GU cancers.15,16 For example, a meta-analysis of four clinical trials showed that patients with CAT had a 38% reduced risk of recurrent VTE if they were treated with a Factor Xa (FXa) inhibitor compared with LMWH.14 However, in this same analysis, a trend for an increased risk of major bleeding was observed with the FXa inhibitors, although this did not reach statistical significance.14 In the Hokusai VTE Cancer and SELECT-D studies, this increase in major bleeding was driven predominantly by patients with GI cancers.15,16 In the CARAVAGGIO trial, the risks of major bleeding were similar between the LMWH and apixaban groups, although Thomas noted that there were fewer patients with upper GI cancers enrolled in this study, compared with the Hokusai VTE Cancer and SELECT-D studies.17 Based on the available evidence, international clinical guidelines now recommend the use of DOACs in patients with CAT, with most stating that DOACs and LMWH are preferred over VKAs.18-22 However, there remains a degree of uncertainty in the guidelines for patients at a high risk of bleeding, such as those with GI or GU cancers. For these patients, guidelines currently recommend using DOACs cautiously,18,20,22 or using LMWH in preference.21
Thomas also highlighted several unmet needs for patients with CAT who are receiving anticoagulation therapy, including the increased risk of bleeding compared with patients without cancer, and the challenges associated with prescribing for patients with brain metastases, renal impairment, low platelets, or poor GI absorption due to nausea and vomiting. Physicians should also consider the impact of other cancer treatments when prescribing anticoagulants for CAT, including drug interactions with anticancer therapies, and the potential for disruption to treatment for surgery. Thomas emphasised that there remains a considerable degree of uncertainty in the field of CAT, as current therapies have limitations, and there are still patients who cannot be managed effectively with currently available treatments. Also, higher-risk patients are often excluded from CAT studies, which means that the results from randomised controlled clinical trials may not always be generalisable to all patients with CAT. With patients with cancer living longer, persistence with anticoagulants is particularly key, and patients with active cancer should be considered for long-term anticoagulation therapy after a venous thromboembolic event. However, adherence to anticoagulants is known to be poor in patients with CAT, and this challenge was recently highlighted in a real-world claims-based study presented at the American Society of Clinical Oncology (ASCO) conference in 2022.23 In this study, the median treatment time was only 3.2 months for DOACs and warfarin, and 1.8 months for LMWH.23 Therefore,patients with CAT received anticoagulants for a markedly short duration compared with that recommended in international guidance. Thomas concluded the presentation with a summary of the significant progress that has been made in the field in recent years. However, she highlighted that this progress mainly relates to efficacy, and the principal issue that still remains is the increased bleeding rates in this population. To address this issue, therapies that target FXI are of increasing interest.
New Antithrombotic Approaches: The Promise of Targeting Factor XI
Paula Bolton-Maggs
Currently available anticoagulants function by either blocking thrombin or FXa (DOACs and heparin), or depleting vitamin K levels and preventing the activation of clotting factors (VKAs). These agents reduce the risk of thrombosis, but with a concomitant increase in bleeding risks, particularly for high-risk patients. For example, in elderly patients with atrial fibrillation, annual rates of major bleeding on DOACs can be as high as 12%.24 As an alternative, novel agents are being developed that target the contact activation system, including therapies that target Factor XII and FXI. These agents are currently being investigated in animal and human studies, with preliminary data showing that reducing FXI activity can reduce the thrombotic risk without increasing the risk of bleeding.25,26
Factor XI is an appealing target for antithrombotic drugs for several reasons. Firstly, congenital FXI deficiency results in only a mild bleeding disorder, and is only associated with bleeding after surgery in areas of high fibrinolytic activity, such as the mouth and GU system.27 Secondly, deficiency of FXI is not associated with spontaneous bleeding.27 Thirdly, there is a minimal relationship between FXI coagulant levels and bleeding risk, but there is an association between high FXI levels and thrombosis.25,27,28 Finally, there is a surprisingly high prevalence of congenital FXI deficiency in the general population across continents, suggesting that there may even be a possible survival advantage to having lower levels of FXI.29
These observations can potentially be explained by emerging data that indicate FXI is an attractive target for new anticoagulants, as it may be critical in thrombosis development but only plays a minor role in the physiological process of haemostasis.30 In haemostasis, FXI acts at the interface between the process of tissue factor-initiated thrombin generation and the contact activation pathway (the kinin–kallikrein system).31,32 In this model, FXI(a) has only a supporting role in haemostasis and does not make a significant contribution if a strong tissue factor signal is present.32 Conversely, there is a growing body of evidence supporting an important role of FXI in thrombosis. To begin with, FXI deficiency has been shown to protect against thrombotic stroke,33 DVT,34,35 and cardiovascular events (composite of myocardial infarction [MI], stroke, and transient ischaemic attacks).35 In addition, in large case-control studies, high levels of FXI were associated with an increased risk of VTE36,37 and MI.38 Finally, treatment of patients deficient in FXI with FXI concentrates has resulted in thrombotic events.39 A role for FXI inhibition in anticoagulation is also supported by animal studies. For example, Factor XII and FXI knockout mice are protected from arterial and venous thrombosis without evidence of bleeding.40,41 Studies with antisense oligonucleotides that reduce the hepatic synthesis of FXI in rodents and monkeys have also demonstrated the prevention of thrombosis without excessive bleeding.42
Bolton-Maggs also discussed the factors that may contribute to the variable bleeding risk observed in individuals with FXI deficiency, including low normal levels of other coagulation factors. Importantly, the capacity for thrombin generation is not related to the activated partial thromboplastin time-based FXI level. To illustrate this, Bolton-Maggs described a patient with congenital FXI deficiency and a history of thrombotic events, including MI and spontaneous DVT. Despite low FXI activity, thrombin generation was similar to a control patient at all tissue factor concentrations, indicating that the concentration of tissue factor rather than the activity of FXI affected thrombin generation (Gillian Pike, personal communication). Bolton-Maggs also explained that thrombin generation tests under specific conditions with low tissue factor concentration could identify patients with FXI deficiency and a history of bleeding from patients with a similar deficiency but no bleeding tendency. In 2015, Pike et al.43 published a study that showed that a thrombin generation assay performed in platelet-rich plasma with the inhibition of the contact activation pathway could identify patients deficient in FXI with a known bleeding tendency.43
Several agents are currently under clinical development that target FXI, including antibodies, small molecules, natural inhibitors, antisense oligonucleotides, and aptamers. Although there are some similarities between these agents, there are also many differences, which may affect their efficacy, safety, and level of patient adherence, which all must be considered, if approved, in the real-world clinical setting (Table 1).25 Results from clinical trials will be required to determine which will be the most appropriate agent to meet a particular clinical need.
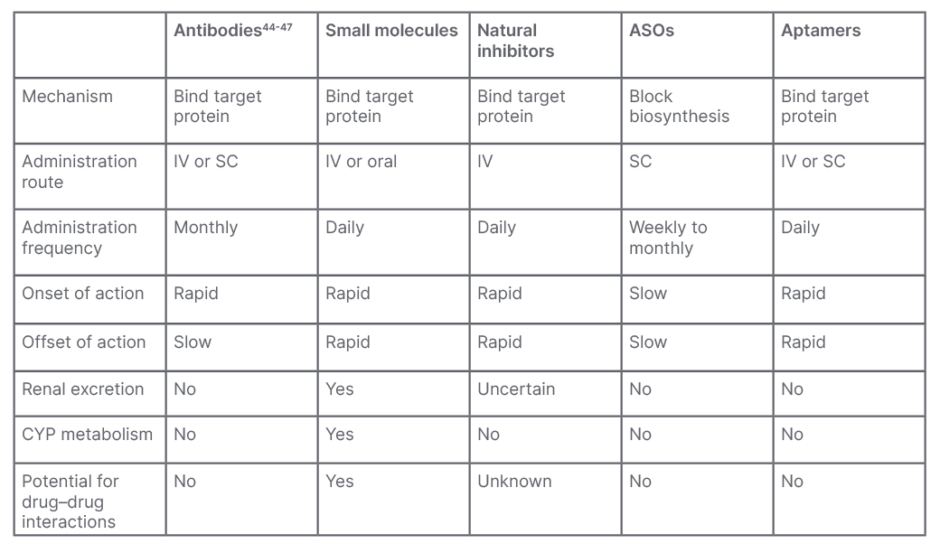
Table 1: Properties of anticoagulant therapies targeting Factor XI.
Adapted from Fredenburgh and Weitz25
ASO: antisense oligonucleotide; CYP: cytochrome P450; IV: intravenous; SC: subcutaneous.
Agents currently in clinical development include abelacimab, a fully human monoclonal antibody that binds to FXI and locks it in the zymogen (inactive precursor) conformation.3 In studies in healthy volunteers, abelacimab treatment was safe and well tolerated, and resulted in robust and sustained prolongation of activated partial thromboplastin time and FXI suppression.44,45 Abelacimab was subsequently studied in a randomised Phase II trial in patients receiving a total knee arthroplasty.3 Patients receiving a total knee arthroplasty are typically the model of choice for proof-of-concept studies of new anticoagulants due to the high bleeding risk associated with this procedure, and the short duration of anticoagulation. In this study, a single intravenous infusion of abelacimab at doses of 75 mg or 150 mg was effective for the prevention of VTE, and showed superiority over daily LMWH (enoxaparin [ Figure 2A ]).3 A low risk of bleeding was observed in all treatment arms in this study (Figure 2B).3 The evidence supporting abelacimab has also been expanded by four pre-clinical in vitro studies presented at ISTH 2022. These studies demonstrated that pharmacodynamic interactions between abelacimab and two commonly used antiplatelet agents, aspirin and ticagrelor, are unlikely;46 that abelacimab had no impact on platelet aggregation in vitro;47 that recombinant Factor VIIa can potentially be used to manage bleeding in patients treated with abelacimab;48 and that abelacimab may be a useful anticoagulant for patients receiving haemodialysis.49
Asundexian is a small molecule oral agent that targets FXI and is currently in clinical development. In a randomised, double-blind, Phase II study (PACIFIC-AF) in patients with atrial fibrillation, asundexian at doses of 20 mg or 50 mg resulted in reduced bleeding when compared with the DOAC apixaban (Figure 2C).7 Although the results from this study were promising, there were several limitations which must be taken into consideration, including the fact that the trial was not powered to test differences in thrombosis rates between groups. In addition, the number of bleeding events was half of the anticipated number, and no major bleeding events occurred. As a result, the magnitude of the effect of asundexian compared with apixaban could not be accurately determined.50 There are several FXI inhibitors in clinical development, and results obtained so far suggest no increased risk of bleeding.3,7
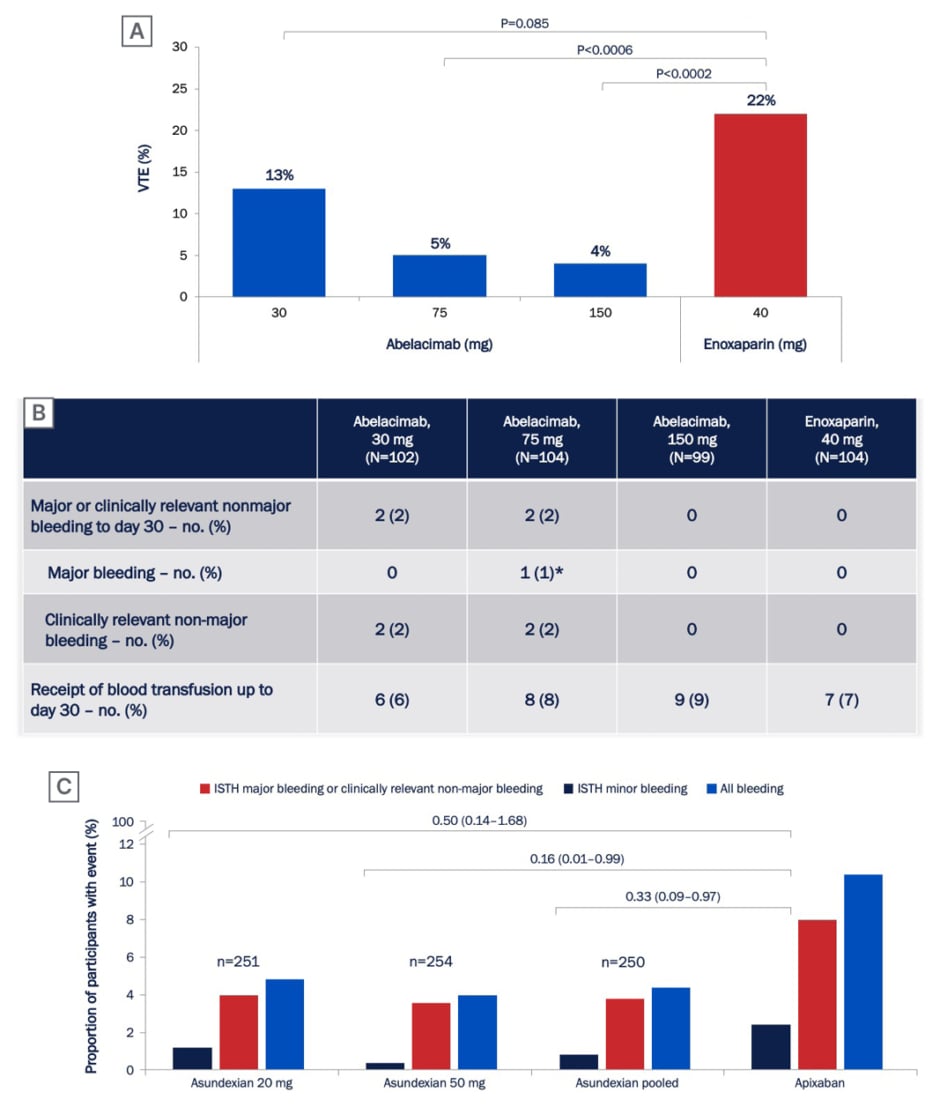
Figure 2: Rates of (A) venous thromboembolism and (B) bleeding in patients receiving abelacimab or enoxaparin after total knee arthroplasty in the ANT-005 TKA Phase II trial,3 and (C) rates of bleeding in patients with atrial fibrillation receiving asundexian or apixaban in the PACIFIC-AF Phase II trial.7
ISTH: International Society on Thrombosis and Haemostasis; no.: number; VTE: venous thromboembolism.
Although clinical data for these investigational FXI inhibitors are promising, Bolton-Maggs cautioned that real-world experience might be different, as patients are very carefully selected for clinical trials. In particular, other factors may influence the capacity for thrombin generation, which may relate to bleeding risk. Furthermore, reversal of FXI inhibition may be required for patients requiring emergency invasive procedures. Although it should be noted that both tranexamic acid and low-dose recombinant FVIIa have been shown to be effective for patients with congenital FXI deficiency with inhibitory antibodies, and therefore a similar strategy may be of value for patients receiving therapeutic FXI inhibitors who are bleeding or undergoing invasive procedures.51 Bolton-Maggs concluded her presentation by emphasising that there is a need for further studies in atrial fibrillation and CAT to provide more information on the efficacy and bleeding risks associated with FXI inhibition in these clinical settings.
Designing Trials for the Treatment of Cancer-Associated Thrombosis
Giancarlo Agnelli
Agnelli began his presentation by acknowledging that DOACs are an effective alternative to LMWH in a large spectrum of patients with VTE and cancer, with the advantage of improved practicality compared with daily administered parenteral therapies. However, unmet clinical needs remain, including reducing the risk of bleeding complications, particularly in those patients with a tumour that originated in the GI tract. Therefore, patient characteristics, including bleeding risk, cancer origin, and comorbidities must be carefully considered when prescribing DOACs.
To address this unmet need, clinical trials should be designed to determine whether it is possible to reduce rates of clinically-relevant bleeding while maintaining the efficacy that has been observed with DOACs. Selecting an appropriate population for a clinical trial of anticoagulants for CAT is critical, as rates of VTE recurrence and bleeding can vary between different cancer sites. In a sub-analysis of the CARAVAGGIO study, the highest rates of VTE recurrence occurred in patients with gynaecological cancer (10.9%) and GI cancer (8.8%).52 The prevalence of major bleeding was greatest in patients with GI or GU cancer (7.2% and 4.8%, respectively).52
Agnelli highlighted the two ongoing Phase III trials from the abelacimab Phase III CAT programme (Figure 3). These two complementary studies are aiming to enrol approximately 2,700 patients across 220 sites in more than 20 countries. This is the largest program of any anticoagulant performed in CAT to date.
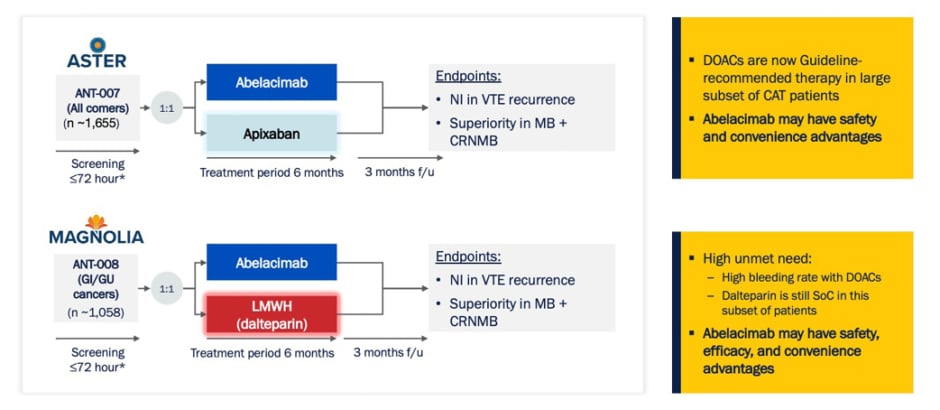
Figure 3: Abelacimab Phase III cancer-associated thrombosis programme.53,54
*SoC treatment during screening.
CAT: cancer-associated thrombosis; CRNMB: clinically relevant non-major bleeding; DOAC: direct oral anticoagulant; f/u: follow-up; GI: gastrointestinal; GU: genitourinary; MB: major bleeding; NI: non-inferior; SoC: standard-of-care; VTE: venous thromboembolism.
The ASTER and MAGNOLIA Phase III trials are international, multicentre, randomised, open-label, blinded endpoint evaluations that have been designed to address a key unmet need for patients with CAT, the risk of clinically-relevant bleeding.53,54 ASTER is designed to compare abelacimab to apixaban in patients with CAT for whom DOAC treatment is recommended.53 In contrast, MAGNOLIA is designed to compare abelacimab to LMWH (dalteparin) in patients with a GI or GU cancer-associated VTE, for whom DOAC treatment is not recommended due to the increased risk of bleeding.54 In both studies, the primary endpoint is the non-inferiority of abelacimab at preventing VTE recurrence, and the secondary endpoint is to show superiority of abelacimab versus apixaban (ASTER) or dalteparin (MAGNOLIA) in the rates of major bleeding and clinically-relevant non-major bleeding.53,54 The comparator arm for both trials represents the current standard of care in their respective patient populations.
Closing Remarks
Gary Raskob
Raskob concluded the symposium by emphasising the large unmet need to reduce the risk of major and clinically-relevant bleeding in patients with CAT, particularly for those with GI or GU cancer. Many of these patients remain inadequately anticoagulated due to the fear of bleeding, and clearly safer and more tolerable antithrombotic strategies are required to reduce the burden of VTE in this population. As an alternative to agents targeting FXa, thrombin, or vitamin K, FXI has recently emerged as an attractive therapeutic target for anticoagulants. Patients with congenital FXI deficiency have been studied for decades, and all the evidence collected to date suggests that these patients are at no higher risk of intracranial or GI haemorrhage than the general population.50 This is reassuring and suggests that FXI inhibitors might be associated with fewer and less life-threatening bleeding episodes. Phase II studies completed to date with FXI inhibitors have yielded promising results, and have shown that FXI inhibition is efficacious at preventing VTE without increasing the bleeding risk.3,7 However, further studies are now needed to confirm these preliminary results. For patients with CAT, the ongoing Phase III ASTER and MAGNOLIA studies are designed to address current unmet needs in this population, including reducing bleeding risk and increasing adherence to therapy. The potential results that may be generated from the ASTER and MAGNOLIA studies has been recently recognised by a regulatory agency, where the U.S. Food and Drug Administration (FDA) has granted fast-track designation to abelacimab as a treatment for CAT, reflecting the large unmet need in this population.55








