Meeting Summary
Artificial intelligence (AI) is a rapidly evolving field in medicine that has the potential to profoundly modify research and patient care. However, implementation of AI techniques is still in the early stages in inflammatory bowel disease (IBD) and the full potential of AI to guide disease management in IBD has yet to be realised.
Crohn’s disease (CD) and ulcerative colitis (UC) are known to be progressive diseases. In eligible patients, early intervention with disease-modifying therapies can slow progression, prevent irreversible damage, and improve long-term patient outcomes. In order to facilitate early intervention, approaches to reduce referral times and allow for timely diagnosis of IBD are needed. Tools such as the Red Flags Index have been developed to aid identification of patients with CD, but AI-based referral and diagnosis tools are likely to emerge in the future.
Tools evaluated in IBD so far have employed AI methodologies to automate image analysis, and although useful for easing clinician workload, these tools are not likely to revolutionise patient care. In the future, AI could be used to analyse large, complex datasets to identify novel patterns and associations between patient and everyday variables and disease outcomes, allowing the prediction of disease course; ultimately, this may reduce the need for regular, invasive testing. Such an approach could lead to a new understanding of the disease, as well as improved patient management in IBD.
Personalised medicine has the potential to improve patient outcomes by tailoring disease management approaches to patient-specific factors; however, further research is needed before personalised medicine can achieve its full potential in IBD. Studies are underway to identify reliable predictors of disease course and treatment response in IBD to allow the development of personalised medicine approaches. Challenges around the implementation of personalised medicine clinical practice will also need to be addressed.
AI approaches have the potential to transform the management of IBD and facilitate a more individualised treatment approach, with the overall aim of improving patient outcomes.
Introduction
This symposium considered the role that AI may play in the future management of patients with IBD. Firstly, Gionata Fiorino highlighted the importance of early intervention and how AI approaches might be used to aid early diagnosis. Edouard Louis subsequently discussed the future role that AI might play in the treatment pathway, using examples from other therapy areas. Finally, Walter Reinisch considered how personalised medicine approaches may transform the management of IBD in the future.
Early Intervention: Fitting the Referral and Diagnosis Pieces Together
Diagnosis of patients with early-stage IBD, especially CD,1 can be challenging due to non-specific symptoms and the lack of a single, non-invasive diagnostic test. As a result, patients with IBD currently experience considerable delays in referral and diagnosis. A survey of 4,670 patients by the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) revealed that although 70% of patients saw a specialist within 1 year of symptom onset, only 54% of patients received a final diagnosis of IBD in this time-frame.2 In addition, 67% of patients needed to attend an emergency clinic more than once before receiving their final diagnosis.2 Delayed referral and diagnosis is a major barrier to early intervention with disease-modifying therapies, particularly in CD, and can lead to disease complications and poor long-term patient outcomes.1,3
The ‘Window of Opportunity’ in Inflammatory Bowel Disease
CD is known to be a progressive disease: bowel damage measured using the Lémann index was significantly greater in patients with longer disease duration.4 The benefit of treating patients with CD within a ‘window of opportunity’, before irreversible tissue damage occurs, is well documented. In a meta-analysis of 1,076 patients with CD treated with adalimumab, remission rates at 6 months and 1 year were significantly higher in patients with less than 1 year disease duration at baseline versus those with longer disease duration.5 Increasing evidence in CD has shown that in eligible patients, early use of disease-modifying agents, such as biologics, slows disease progression, reduces tissue destruction, and leads to improved long-term outcomes, including prevention of hospitalisation and need for surgery.3,6-8
UC is also now considered to be a progressive disease,3,9 and the concept of a ‘window of opportunity’ is emerging in UC. In a pooled analysis of patients with UC treated with infliximab in the ACT-1 and ACT-2 trials, early achievement of mucosal healing (at Week 8) was associated with improved outcomes after 1 year of treatment.10,11 However, ACT-1 and ACT-2 did not specifically assess patients with early disease,11 so further studies are needed to explicitly demonstrate the benefits of early intervention in patients with UC.
The Red Flags Index
Tools have been developed to try to reduce the time to diagnosis and facilitate early intervention in patients with IBD; one such tool is the Red Flags Index for CD.1 This index includes eight items that are independently associated with a diagnosis of CD (Figure 1).1 A prospective, observational study was performed to validate the Red Flags Index.12 The Red Flags questionnaire was administered by general practitioners (GPs) to 112 patients with suspected CD; these patients were then referred to the nearest IBD centre to confirm or exclude CD diagnosis. Overall, 59% of patients completed the visit with the gastroenterologist and four patients were subsequently diagnosed with CD, with three classified as having early disease.
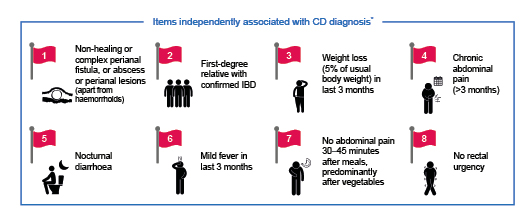
Figure 1: Red Flags Index for the diagnosis of Crohn’s disease.1*A Red Flags Index score of ≥8 was highly predictive of CD diagnosis. CD: Crohn’s disease; IBD: inflammatory bowel disease.
The index had a sensitivity of 50% and a specificity of 58%.12 However, combining the Red Flags Index with measurement of faecal calprotectin significantly improved diagnostic accuracy, giving a sensitivity of 100% and a specificity of 72%.12 The results from this study demonstrate the value of combining clinical symptoms and biomarkers to aid in the early referral and diagnosis of CD.
In the future, AI approaches may further improve the efficiency of referral and diagnostic tools in IBD. AI could be used to automate the analysis of electronic health records to identify patients who may fit the criteria for a diagnosis of CD using the Red Flags Index. Similarly, AI could be used to identify items in electronic health records associated with a later diagnosis of UC, allowing development of a Red Flags Index specifically for UC. Further refinement of the Red Flags Index could also be carried out using AI, to identify the minimum inputs needed for accurate diagnosis.
What Can Artificial Intelligence Bring to the Patient Management Puzzle?
AI is a rapidly evolving field in healthcare, which has the potential to profoundly modify research and patient care;13 AI techniques have been used with success in many areas of medicine. One recent example is drug repurposing for the treatment of COVID-19. An AI algorithm was used to search a large repository of medical information to identify approved drugs that might block the severe acute respiratory syndrome coronavirus-2 viral infection process.14 The algorithm predicted that baricitinib, a JAK inhibitor, may reduce the ability of the virus to infect cells in the lungs.14 In a subsequent clinical study, baricitinib combined with remdesivir, an antiviral agent, was found to be superior to remdesivir alone in reducing recovery time among hospitalised patients with COVID-19.15
AI approaches are also being used in IBD, although the use of AI in IBD is not as advanced as in other fields of medicine (Figure 2). Research has progressed from initial exploratory proof-of-concept studies, through to the development of time-saving AI-based tools, such as algorithms capable of automated image analysis. Algorithms to help guide clinical decisions are beginning to be investigated; however, the overall goal of AI, which is to help facilitate a fully personalised treatment approach to improve patient outcomes, has still not been reached.
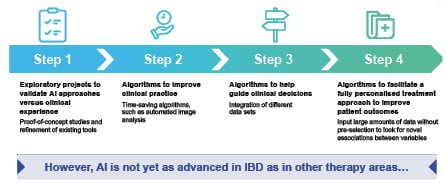
Figure 2: The evolution of AI in inflammatory bowel disease.
AI: artificial intelligence; IBD: inflammatory bowel disease.
Artificial Intelligence in Image Analysis in Inflammatory Bowel Disease
To date, AI techniques in gastroenterology have mostly been used to aid analysis of images generated during endoscopy procedures. A feasibility study showed that a deep-learning algorithm could be used to detect and localise colonic polyps in real-time with an accuracy of 96.4%.16 This system could be used to improve the adenoma detection rate for the prevention of colorectal cancer.16 In CD, AI has also been used to help automate the analysis of capsule endoscopy imaging. Capsule endoscopy is a valuable tool for the diagnosis of CD, but analysis is tedious and requires the clinician to manually identify several views of the same lesion from a large amount of video footage, which can be difficult. A machine-learning algorithm was able to automatically identify images of the same lesion with an accuracy of 88%, thus reducing burden for the clinician.17 A deep-learning algorithm has also been used in UC to evaluate inflammation severity in colonoscopy images.18 This algorithm was able to differentiate colonoscopy images of varying Mayo endoscopic scores with a high degree of accuracy, and could act as a companion tool during endoscopy.18 These time-saving AI algorithms will improve clinical practice; however, they will not revolutionise patient care and only scratch the surface of what AI is capable of. So, what should be the aim of AI in IBD?
Future Use of Artificial Intelligence in Inflammatory Bowel Disease
The potential for AI in IBD extends much further than automation of image analysis. In the future, AI could be used to analyse vast amounts of unselected data on patient variables and disease outcomes to identify complex patterns and associations that are beyond the scope of the human brain. Such an approach could lead to a new understanding of disease and improved patient management in IBD. For example, could AI be used to help find novel associations between everyday patient variables and disease outcomes in IBD? AI could be used to integrate a broad range of patient data collected during everyday life, such as blood pressure, sleep cycles, and physical activity levels, to predict disease evolution in real time. Tools based on these analyses could help to avoid the need for regular invasive testing.
Metabolomic analysis is another area where AI is likely to play a role in the future. Metabolomics is the analysis of small molecular metabolites in biological samples such as urine, stool, blood, and tissue biopsies. Due to the complexity of the data, AI techniques such as machine- learning are often used for data interpretation.19 Metabolomics has already been used to provide insights into IBD pathogenesis,19 and the potential exists for metabolomics to allow enhanced patient stratification for clinical management. Could metabolomic analysis of stool samples be used to diagnose IBD or predict relapse in the future?
There are many steps in the patient journey where AI could be employed to improve the management of IBD, but pressing unmet needs are currently reducing referral times and prediction of response or relapse to a given therapy. Identification of early markers of disease (pre-Red Flags) could aid a person to self-refer to a GP and other variables measured in everyday life could signal a GP to refer the patient to a gastroenterologist. Once diagnosis is confirmed, AI could be used to predict response to treatment prior to initiation of a given therapy or monitor a patient once therapy has commenced for markers that may predict relapse of disease, facilitating a fully personalised treatment approach. The era of personalised medicine is about to dawn in IBD, and AI techniques could allow its full potential to be realised.
The Coming-of-Age of Personalised Medicine in the Inflammatory Bowel Disease Clinic
Personalised medicine is the tailoring of disease management approaches based on patient-specific factors to help improve patient outcomes. Factors assessed can include genetics, epigenetics, and the microbiome, as well as clinical and lifestyle factors.20 Personalised medicine is of particular value in multiple-hit diseases such as IBD, where patients can be highly heterogenous due to the complex interplay between genetic and environmental factors that drives the pathogenesis of the disease.20,21 Long-term disease management can be difficult in IBD and there is a need for personalised medicine to transform disease management by allowing selection of the most effective therapy for each individual patient (Figure 3). However, implementation of personalised medicine remains a challenge in IBD, as reliable predictors of disease course and treatment response are lacking.
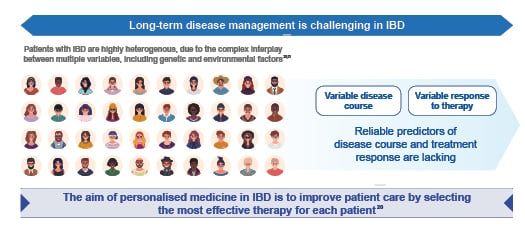
Figure 3: The need for personalised medicine in inflammatory bowel disease.20,21
IBD: inflammatory bowel disease.
In IBD, some aspects of disease management in routine clinical care are already governed in part by personalised medicine. Patient and disease characteristics are already used to guide clinical decision-making,22 and biomarkers of inflammation, such as faecal calprotectin and C-reactive protein, can be used to predict the course of the disease23,24 and monitor treatment response.22 Therapeutic drug monitoring can also be used to guide dose adjustments and optimise outcomes.25
Clinical Decision Support Tools
Clinical decision support tools based on commonly collected clinical data on patient and disease characteristics are in development for IBD to help predict response to biologic therapies.26,27 A clinical decision support tool was developed to identify factors associated with clinical, steroid-free, and durable remission following treatment with vedolizumab in patients with CD included in the GEMINI 2 trial.26 Multivariable logistic regression was used to identify variables associated with remission, including no prior bowel surgery, no prior anti-TNF exposure, and no prior fistulising disease, as well as baseline albumin and C-reactive protein levels. In the final prediction model, a points system was developed to give a weighting to each variable. This final model was then validated in an independent cohort of patients treated with vedolizumab (the VICTORY cohort). A cut-off value of 13 points identified patients in clinical remission after vedolizumab with 92% sensitivity; patients in steroid-free remission with 94% sensitivity; and patients with mucosal healing with 98% sensitivity.26 Multivariable logistic regression was also used in a separate study to develop a matrix-based tool to predict primary non-response to infliximab in patients with CD. Three simple clinical factors were included in the final predictive model: age at first use of infliximab, BMI, and previous surgery. The model demonstrated good accuracy, with an area under the receiver operating characteristic curve of 0.8 (95% confidence interval: 0.67–0.93).27 In the future, AI techniques could be used to integrate further variables predictive of response and improve the accuracy and clinical applicability of these tools.
Biomarkers of Prognosis and Response
Despite these advances, further research is needed before personalised medicine can achieve its full potential in IBD. Prognosis at diagnosis and prediction of response to targeted treatments are key stages in the IBD treatment pathway where advances in personalised medicine could help to improve patient outcomes. Studies are currently being carried out to investigate the potential for omic datasets, including genomics, the microbiome, and cellular composition data, to inform personalised medicine approaches and improve patient stratification.
Genomic risk scores developed using genome-wide single nucleotide polymorphism data could play a future role in disease prognosis. In a study from the International IBD Genetics Consortium, which included >68,000 patients with IBD and 29,000 healthy controls, genomic risk scores were predicted based on single nucleotide polymorphisms. The genomic risk score was found to be associated with disease severity in patients with CD.28 Patients with higher predicted genomic risk scores had clinical characteristics typically associated with a more severe disease course, including requirement for bowel resection (p<0.03), younger age at onset (p<0.005), and ileal disease location (p<0.003).28 Analysis of the gut microbiome also offers potential for identification of predictive biomarkers for disease progression. In one study, complex associations were identified between multi-omic components of the gut microbiome and host that may influence disease activity in IBD.29
Biomarkers of treatment response are also being investigated in IBD. Novel markers of anti-TNF response have been studied in two single-cell analysis studies. In the first study, analysis of inflamed tissue from patients with CD allowed identification of a unique cellular module, consisting of IgG plasma cells, inflammatory mononuclear phagocytes, activated T cells, and stromal cells, that was significantly associated with anti-TNF response.30 In the second study, analysis of colon tissue from patients with UC revealed that inflammatory cell signatures from pre-treatment samples may be associated with future anti-TNF response.31 While a range of potential biomarkers of response have been identified in preliminary studies in IBD, none have yet been validated for use in the clinic. Appropriately powered studies are required to validate potential biomarkers of response.
Challenges Surrounding the Implementation of Personalised Medicine in Inflammatory Bowel Disease
In addition to the lack of validated predictive biomarkers, there are several other challenges surrounding the optimisation and implementation of personalised medicine tools in IBD. Research is needed to further optimise diagnostic criteria before personalised medicine and AI approaches can be used to aid diagnosis. In addition, personalised medicine will require the development of definitions of response that are rooted in the biology of the disease, rather than in patient-reported symptoms. Consensus opinions are also needed on reproducible patient characteristics that can be used to inform personalised medicine approaches, as well as the most appropriate time to assess treatment response.
Personalised Medicine in Other Therapy Areas
Despite similar challenges, personalised medicine approaches have been implemented successfully in other therapy areas including oncology, where a companion diagnostic test to predict response to a targeted therapy has been developed in non-small cell lung cancer. The KEYNOTE-001 study identified a clear link between the efficacy of pembrolizumab, a monoclonal antibody that targets the programmed cell death protein (PD-1), and tumour expression of the PD-1 ligand (PD-L1). Based on these data, pembrolizumab is only indicated in patients who have a tumour that expresses PD-L1 above a specific level, as determined by the companion diagnostic assay.32 Another example is in rheumatoid arthritis, in which an exploratory study has used machine learning to develop a biomarker panel to predict non-response to anti-TNF.33 Development of a similar tool in IBD would be extremely useful for the advancement of personalised medicine.
Concluding Remarks
AI techniques are emerging in IBD, but further study is needed to allow these tools to reach their full potential in the management of disease. Initial data in IBD have shown that AI-based tools can be used to ease clinician workload by analysing images or cumbersome datasets. However, AI also has the potential to improve patient outcomes by facilitating early referral and diagnosis, as well as predicting prognosis and response to treatment. The era of personalised medicine is only just dawning in IBD. Clinical decision support tools are in development and novel biomarkers of treatment response have been identified, but these findings need to be validated before they can be used in the clinic to improve patient outcomes. AI techniques may help to accelerate the search for appropriate personalised medicine tools in the future, but standardisation of various parameters in disease management is needed before the era of personalised medicine in IBD can truly begin.








