Meeting Summary
This year-in-review article provides insights into clinical updates relating to granulocyte-colony stimulating factor (G-CSF) biosimilars research presented at five key congresses in 2022. These include the American Society of Clinical Oncology (ASCO) 55th Annual Meeting (3rd–7th June 2022, Chicago, Illinois, USA), European Society for Medical Oncology (ESMO) Congress (9th–13th September 2022, Paris, France), ESMO Asia Congress (2nd–4th December 2022, Singapore), San Antonio Breast Cancer Symposium (SABCS; 6th–10th December 2022, Texas, USA), and American Society of Hematology (ASH) 64th Annual Meeting and Exposition (10th–13th December 2022, New Orleans, Louisiana, USA). Alongside reviewing the current research presented at these key congresses, with a focus on the use of G-CSF agents and biosimilars in patients undergoing treatment for breast, colorectal, and gynaecological cancers, this article provides an overview of current guidelines on the use of G-CSF in supportive cancer care to manage chemotherapy-induced febrile neutropenia and explores trends in G-CSF biosimilars research.
INTRODUCTION
Chemotherapy-induced neutropenia is a frequent, severe, adverse haematological complication of myelosuppressive and immunosuppressive cytotoxic chemotherapy treatment.1-5 Chemotherapy-induced neutropenia is defined by the National Comprehensive Cancer Network (NCCN) guidelines as a low white blood cell count (neutrophils), which can result in immunosuppression and is associated with an increased risk of infection that, when accompanied by fever, is termed febrile neutropenia.5,6 Patients undergoing cytotoxic chemotherapy are, therefore, at a greater risk of contracting a life-threatening infection.
The occurrence of febrile neutropenia is associated with the intensity of chemotherapy and remains one of the most frequent and serious complications of chemotherapy.1-5 Development of febrile neutropenia may compromise the treatment response, as it may necessitate dose reduction and treatment delay.7,8 This imposes a substantial economic burden,7,8 requiring hospitalisation and treatment with broad-spectrum antibiotics, and is associated with significant morbidity and mortality.3,6 In patients with early breast cancer, neutropenia is reported to be the most common adverse event experienced, resulting in a reduction in relative dose intensity (RDI) for those receiving chemotherapy, reducing treatment efficiency and overall survival.9
A survey in the USA, presented during a poster session at ASH 2022, investigated the National Inpatient Sample (NIS) database in 2019 for the prevalence and clinical outcomes of febrile neutropenia in patients hospitalised with cancer.10 Out of 118,965 patients hospitalised for febrile neutropenia or fever and neutropenia, 24,444 patients (88.1%) had a co-diagnosis of cancer and febrile neutropenia, including 24.5% (n=5,995) who were paediatric patients (<18 years). The severity of illness and risk of mortality was classified as ‘moderate’ in 57.2% (using the All Patient Refined Diagnosis Related Group [APR DRG] severity index). Among this sample of inpatients hospitalised with febrile neutropenia, the most common cancer types were haematological malignancies (59.4%), including non-Hodgkin lymphoma, acute myeloblastic or lymphoblastic leukaemia, myelodysplastic syndrome, and multiple myeloma. Other cancer types included breast cancer (7.8%) and gastrointestinal cancer (5.0%).10
GUIDELINES FOR GRANULOCYTE-COLONY STIMULATING FACTOR PRIMARY PROPHYLAXIS AND TREATMENT OF FEBRILE NEUTROPENIA
International febrile neutropenia guidelines from the European Organisation for Research and Treatment of Cancer (EORTC),1 ASCO, and Infectious Diseases Society of America (IDSA) joint update,2,3 ESMO,4 as well as the NCCN,5 aim to standardise the recommendations for the prevention, diagnosis, and treatment of febrile neutropenia (Table 1). The overall consensus is that febrile neutropenia is indicated by a fever above 38 oC, accompanied by an abnormally low absolute neutrophil count (<0.5×109/L) and may also present with signs of infection, typically identified by physical examination, blood and urine cultures, and imaging, where necessary.1-5
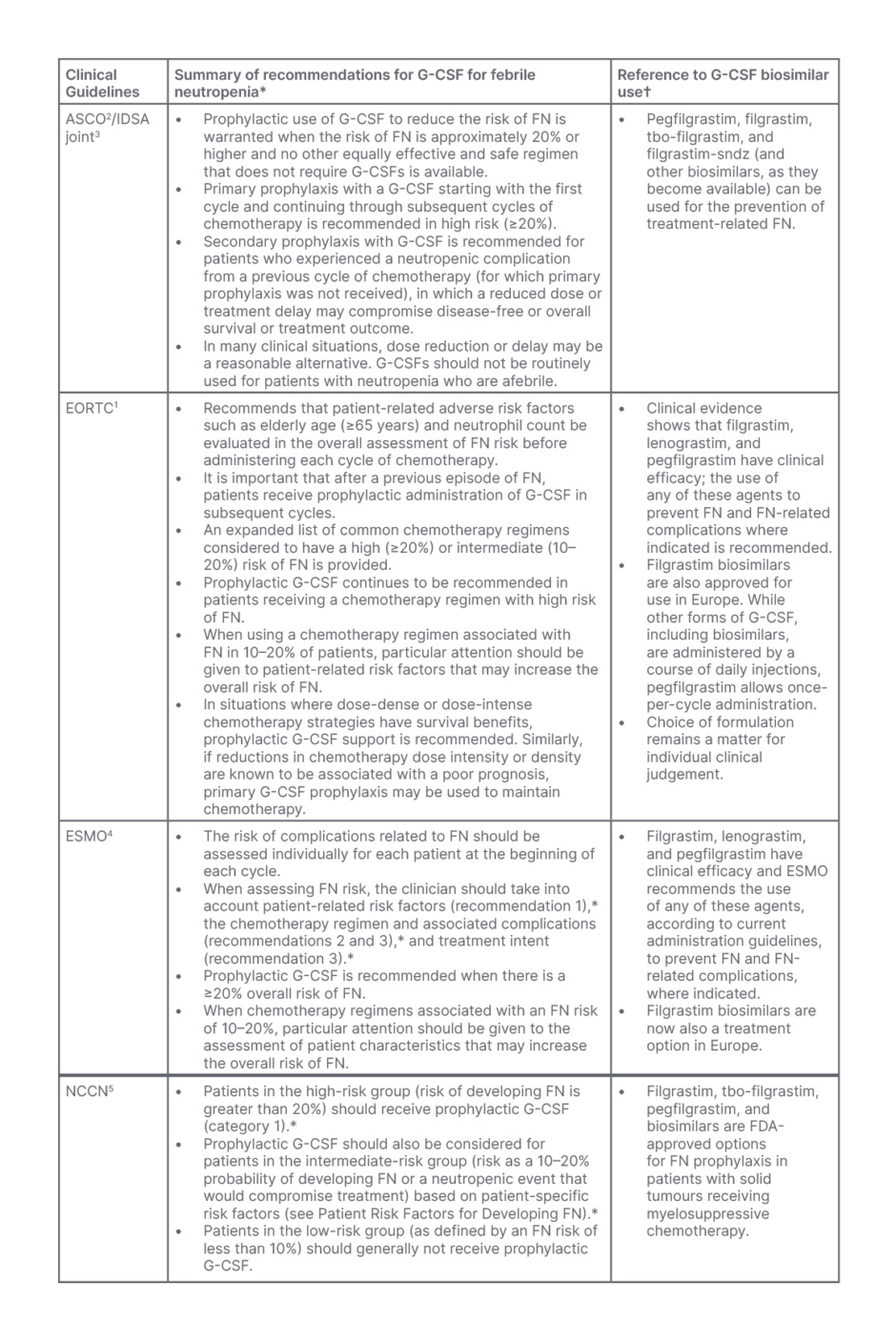
Table 1: Summary of the clinical practice guidelines for adult patients with cancer at risk of, or who require treatment for febrile neutropenia.1-5
*Please refer to the complete guidelines for detailed information, recommendations, and further clinical tools and resources.
†Approval may have been granted from either the EMA or FDA but may not be authorised in all countries, and some agents may have been added or withdrawn from approval at the time of print.
ASCO: American Society of Clinical Oncology; ECORTC: European Organisation for Research and Treatment of Cancer; EMA: European Medicines Agency; ESMO: European Society for Medical Oncology; FDA: U.S. Food & Drug Administration; FN: febrile neutropenia; G-CSF: granulocyte-colony stimulating factor; IDSA: Infectious Diseases Society of America; NCCN: National Comprehensive Cancer Network.
Due to the high risk and frequency of febrile neutropenia associated with chemotherapy, current guidelines recommend analysing the risk before each chemotherapy cycle regimen,4 and considering whether the use of primary prophylaxis to prevent chemotherapy-induced febrile neutropenia may be warranted.1-5 The Multinational Association of Supportive Care in Cancer (MASCC) prognostic index is widely used to predict the prognosis of febrile neutropenia after chemotherapy in patients with cancer,11 as well as the Clinical Index of Stable Febrile Neutropenia (CISNE) score.12
Primary prophylaxis is based on the use of G-CSF to stimulate granulocyte proliferation in the bone marrow and increase the number of circulatory neutrophils, supporting haematopoietic recovery following chemotherapy.5,13 G-CSF may be used as supportive care for both solid and haematological tumours, typically administered 24–48 hours after the first cycle of chemotherapy.14
According to the ASCO/IDSA, EORTC, ESMO, and NCCN practice guidelines (Table 1),1-5 G-CSF agents, including filgrastim or pegfilgrastim, and several biosimilar products associated with the reference (originator) drug (such as tbo-filgrastim, filgrastim-sndz, filgrastim-aafi, pegfilgrastim-jmdb, pegfilgrastim-bmez, and pegfilgrastim-apgf), are also approved for the reduction of neutropenia-related outcomes.15 (These drugs may have been approved by either the European Medicines Agency [EMA] and/or the U.S. Food and Drug Administration [FDA] and may not be authorised in all countries, and some may have been added or withdrawn from approval at the time of print).
A retrospective, non-randomised, non-interventional study presented at ASCO 2022 presented data on the use of G-CSFs in clinical practice in Russia.7 A total of 492 patients with solid tumours receiving myelosuppressive chemotherapy were included in the analysis. The majority of patients presented with breast (68%), colorectal (10%), and urogynaecological cancer (10%). In total, 30% of patients had at least one risk factor for febrile neutropenia and 14% had a high risk of febrile neutropenia based on the chemotherapy regimen. Primary prophylaxis was administered to 44% of patients with a high risk of febrile neutropenia, with 25% receiving filgrastim and 19% empegfilgrastim. A total of 3% of patients developed febrile neutropenia. However, 8% of patients had a reduction in their chemotherapy dose by 20–25%, and second-cycle chemotherapy was delayed in 27% of patients, suggesting that oncologists in Russia continue to use dose reduction or dose delays in clinical practice. Adherence to guidelines regarding use of primary prophylaxis is, therefore, poor.7
Practice Recommendations for the Use of Granulocyte-Colony Stimulating Factor for Febrile Neutropenia
Practice guidelines recommend the use of primary prophylaxis of G-CSF based on individualised patient-specific risk factors and the overall risk of developing febrile neutropenia by chemotherapy regimen (high [≥20%], intermediate [10%–20%], or low [<10%] risk [Table 1]).1-5
Typically, the use of G-CSF as a primary prophylactic is recommended in chemotherapy regimens when there is a high risk (≥20%) of febrile neutropenia, or for patients who receive chemotherapy with additional risk factors that place them at intermediate risk (>10% but less than <20%).1-5 Risk factors are based on patient-, disease-, and treatment-related factors such as being elderly (aged 65 years and over), the presence of severe symptoms such as hypotension or invasive fungal infections, and these should be determined to establish if overall risk places them as high or low.1-5 In those who are at low risk for febrile neutropenia (<10%), primary prophylaxis with G-CSF is not indicated.1-5 Secondary prophylaxis is recommended following febrile neutropenia or when there is a dose-limiting neutropenic event. Off-label use of G-CSFs for afebrile neutropenia is not recommended.2
Interim Updates to the Granulocyte-Colony Stimulating Factor Primary Prophylaxis Guidelines for Febrile Neutropenia
Recently, in view of the COVID-19 pandemic and the potential risk of infection, multiple organisations (ASCO, ESMO, and NCCN) issued interim modifications to existing guidelines and recommendations to extend the use of G-CSF prophylaxis to include patients at intermediate-risk (10–20%) of febrile neutropenia, regardless of additional risk factors.16-18
In light of this, real-world evidence was presented at ESMO 2022, describing retrospective data from patients with solid tumours, including gastrointestinal (32.2%), lung (20.7%), and breast (7.8%) cancer, receiving anti-neoplastic chemotherapy prior to and during the COVID-19 pandemic (January 2019–December 2021; N=1,666) in Israel.19 Rates of febrile neutropenia remained stable (2.45% pre-pandemic versus 2.35% during the pandemic; p=0.898), although, consistent with interim guidelines, more patients received G-CSF during the pandemic (16.00% versus 6.00% pre-pandemic; p<0.0001), and 1-year survival was higher during the pandemic period (p=0.021).19 An ambispective, observational study, presented at SABCS 2022, indicated that patients with early breast cancer who had undergone neoadjuvant chemotherapy (N=183) in Spain, resulted in 13% being admitted to hospital for COVID-19 during March 2020 and May 2022, and found 41% (n=9) had received G-CSF. However, there was no association between the use of G-CSF and the occurrence of symptomatic COVID-19 infection, despite modified G-CSF guidelines being proposed to deter potential risk.20 Therefore, the interim guidelines may need to be reviewed.
RECENT EVIDENCE FOR BENEFITS OF GRANULOCYTE-COLONY STIMULATING FACTOR PROPHYLAXIS
The use of G-CSFs has proven efficacy in the prophylaxis of chemotherapy-induced neutropenia and is associated with reducing the incidence of febrile neutropenia and hospitalisation.8
A Phase II clinical trial of adverse events in patients with human epidermal growth factor receptor2-positive (HER2+) metastatic breast cancer was presented at ESMO 2022.21 The study reported that the occurrence of Grade ≥3 treatment-emergent adverse events of a decrease in white blood cell count and/or decrease in neutrophil count was less common in patients who had received G-CSF prophylaxis (23.3%; n=10/43), compared to those without G-CSF prophylaxis (36.1%; n=13/36).21
Interim results from a post-registration, prospective study that evaluated the impact of primary prophylaxis with empegfilgrastim on the ability to complete planned neoadjuvant chemotherapy courses in patients with early breast cancer (N=195) were presented at SABCS 2022.9 An RDI of >90% was achieved in patients receiving dose-dense regimens for HER2+, hormone receptor-positive/HER2-negative or triple-negative breast cancer, with only one case of neutropenia (0.9%; n=1/111) leading to a drop in RDI in a patient with HER2+ breast cancer. Preliminary results suggest this may improve therapeutic efficacy, as pathological complete response rates in this study were higher than those seen in a historical control population; further data from this trial are anticipated.9
Regarding optimal therapy, a multicentre, prospective, observational, post-registration study conducted in Russia looked at the use of prolonged G-CSF empegfilgrastim in patients with solid tumours (N=2,000), including breast cancer (n=350), colorectal cancer (n=63), and gynaecological tumours (n=35).22 The study investigated the RDI of a single agent in at least one cycle of chemotherapy-based regimen combined with empegfilgrastim. While 22.4% (n=118) of patients had dose delays or dose reductions, only 1.1% (n=6) cited neutropenia as the reason for reduced RDI. The real-world evidence demonstrated that primary G-CSF prophylaxis with prolonged empegfilgrastim administration effectively maintained RDI ≥85% in the routine clinical practice of patients with solid tumours, including 96.3% of patients with breast cancer, 92.1% of patients with colorectal cancer, and 82.9% of patients with gynaecological tumours.22
A further study conducted in Spain between 2015–2021 investigated the efficacy of using filgrastim G-CSF prophylaxis as a useful and safe option in the neoadjuvant setting for HER2-negative breast cancer.23 In total, 204 patients, including those with HER2+ (64%) and triple negative (25%) tumours, were included. Overall, G-CSF prophylaxis facilitated treatment compliance. Febrile neutropenia was reported in 18.1% of patients, with no significant difference in incidence between patients receiving pegfilgrastim or filgrastim (p=0.99). The outcomes indicated that although G-CSF facilitated treatment compliance, a risk of neutropenia was still present.23
An oral presentation delivered at ESMO Asia 2022 presented data from a real-world study in Nepal comparing filgrastim to single-dose pegfilgrastim in a cohort of patients with solid tumours (N=112) who were receiving high-risk or intermediate-risk chemotherapy regimens for febrile neutropenia.14 The study found that febrile neutropenia was significantly lower with pegfilgrastim (43%) compared to filgrastim (70%), along with a reduction in hospitalisation. Overall, primary prophylactic treatment with G-CSFs (filgrastim and pegfilgrastim) minimised hospital stays, visits, and the frequency of admission in patients with cancer receiving chemotherapy. The authors concluded that patients require an adequate evaluation and determination of prophylactic G-CSFs to optimise their clinical care outcomes and reduce hospitalisation-related morbidities through the management of febrile neutropenia.14
GRANULOCYTE-COLONY STIMULATING FACTOR BIOSIMILARS ARE COMPARABLE TO THEIR REFERENCE PRODUCTS
Biosimilars have been approved for use in oncology as an alternative to reference products, as long as they have been approved for the registered indication.15 Biosimilars offer increased accessibility to biologics without the financial implications to patients and the healthcare systems, and represent a safe, effective, and cost-effective alternative to reference products.24 ASCO supports that biosimilars and reference products are considered equally efficacious for the purpose of inclusion in ASCO clinical practice guideline recommendations,25 while ESMO recognises the potential of biosimilars to help achieve sustainable treatment in oncology, providing the manufacturing of biosimilars adheres to the stringent regulations and guidelines set out by regulatory authorities and the World Health Organization (WHO).15
Several filgrastim biosimilars were approved and have entered the USA market from 2015, followed by pegfilgrastim biosimilars in 2018.24 Examples of current G-CSF biosimilars include filgrastim-sndz, tbo-filgrastim, filgrastim-aafi, pegfilgrastim-jmdb, pegfilgrastim-cbqv, pegfilgrastim-bmez, pegfilgrastim-apgf, and pegfilgrastim with on-body injector.
Granulocyte-Colony Stimulating Factor Biosimilar Use for Supportive Cancer Care
A health services survey conducted in the USA was presented at ASCO 2022.26 The survey examined current trends in biosimilar use in oncology pharmacy practice. Results demonstrated a notable shift towards the use of biosimilars compared with the reference product.26 The survey identified an 88% average utilisation of filgrastim and 55% average utilisation of pegfilgrastim, with 90% of the surveyed pharmacies indicating that they had a preferred biosimilar on the formulary. The key barrier to biosimilar adoption was associated with insurance reimbursement, and the survey identified that opportunities still exist to align formularies and consider the use of biosimilars to promote safe and cost-effective care.26
At SABCS 2022, a poster presentation provided an insight into population-based data from patients with breast cancer, comparing G-CSF biosimilar use with the reference products.24 A retrospective analysis of data acquired from the Biologics and Biosimilars Collective Intelligence Consortium (BBCIC) distributed research network database of administrative claims for prophylactic G-CSF products between 2015–2019 was performed. Inclusion criteria included patients with breast cancer over the age of 20 years who were receiving their first cycle of chemotherapy. Of the patients included (N=11,788), 0.8% were male and 49.0% were aged 50–64 years. Based on those patients administered intravenous chemotherapy drugs, 93.0% (n=10,953) had a high risk for febrile neutropenia.24 In addition, other risk factors associated with an increased risk of febrile neutropenia included 7.2% (n=850) with severe hepatic disease (i.e., hepatitis, non-alcoholic steatohepatitis, cirrhosis, and fibrosis), 3.2% (n=373) had open wounds, and 3.1% (n=371) had received surgery within 6 months prior to the first cycle of chemotherapy. Additionally, 7.0% (n=821) had used antibiotics during their first cycle of chemotherapy.
Febrile neutropenia events were low, occurring in 1.8% (n=218) of patients during their first chemotherapy cycle. Serious allergic reactions occurred in 6.7% (n=786) and other adverse events were observed in 4.1% (n=485). The majority of patients received a reference product, pegfilgrastim (92.0%; n=10,895), including 93.0% (n=10,162 out of 10,953) of those receiving high-risk chemotherapy. For biosimilar use, 3% received pegfilgrastim-cbqv, 2% pegfilgrastim-jmdb, 1% filgrastim-sndz, and <1% tbo-filgrastim or a combination of filgrastim plus a biosimilar. Despite guidelines recommending the use of G-CSF for high-risk chemotherapy,1-5 85% of patients who received low-risk chemotherapy (n=25) received pegfilgrastim. The administration of a reference G-CSF reduced from 97% to 76% between 2015–2019. By 2019, biosimilar administration had increased, with 13% having been administered pegfilgrastim-cbqv, and 8% pegfilgrastim-jmdb. The administration of reference products of filgrastim (n=156) decreased over 2016–2019 (3% versus <1%, respectively); however, in general, there was a low administration of tbo-filgrastim (n=<10), and 1% filgrastim-sndz. The increased use of pegfilgrastim biosimilars (2018 onwards) corresponded with the introduction of market availability.24 This marks a slow but positive shift in the uptake of G-CSF biosimilars, but demonstrates that further opportunity exists.
Also presented at ASCO 2022 was a retrospective cohort study of patients in the USA receiving either the reference G-CSF or biosimilar G-CSF between 2018–2021.8 The risk of hospitalisation for febrile neutropenia was compared between reference and biosimilar groups. Of the patients included (N=13,670), 49.2% were receiving a biosimilar G-CSF and the other half received a reference G-CSF.8 Despite pegfilgrastim being the most common G-CSF medication overall (73.5%), filgrastim was used more commonly in the biosimilars group (43.2% versus 10.4% for pegfilgrastim; p<0.05). Of those receiving a G-CSF biosimilar, the key characteristics indicated that they were usually younger, male, had more G-CSF claims, and were more likely to use a specialist pharmacy.8 The incidence of hospitalisation for febrile neutropenia was low (1.1% of patients), with no significant difference between biosimilar and reference products (1.0% versus 1.1%; p=0.39). This study, therefore, demonstrated similar effectiveness of G-CSF biosimilars in preventing hospitalisation due to febrile neutropenia in comparison with the reference G-CSF product.8
EMERGING TOPICS IN GRANULOCYTE-COLONY STIMULATING FACTOR PROPHYLAXIS RESEARCH
The method of delivery of G-CSF biosimilar pegfilgrastim-cbqv was the focus of a poster presented at ASCO 2022.27 Pegfilgrastim-cbqv is normally administered via prefilled syringe 24–72 hours after a patient receives chemotherapy, necessitating a separate clinic visit. Delivery via an on-body injector applied on the day of chemotherapy would eliminate this need. A Phase II, open-label, cross-over study assessing the pharmacokinetic and pharmacodynamic bioequivalence and safety of pegfilgrastim-cbqv found that geometric mean ratios for pharmacokinetic and pharmacodynamic parameters for the two modes of administration fell within range, indicating bioequivalence.27 Similar safety and immunogenicity profiles between the two administration methods were demonstrated.27 This study highlights a potential future approach to the administration of G-CSF biosimilars.
Furthermore, the timing of G-CSF administration was the focus of another poster presented at ESMO 2022.28 The real-world evidence of G-CSF administration within 3 days in patients with breast cancer treated with chemotherapy, found those who received prophylactic G-CSF during their first cycle were highly effective in preventing febrile neutropenia in the following 3 weeks.28 However, the authors indicated that the patients were largely unprotected during the first week of chemotherapy, with results demonstrating that the majority of febrile neutropenia occurred in the first week (81%; n=440 out of 546).28 This has led to emerging insights into the use of a combination-therapy of pegfilgrastim with a novel chemotherapy-induced neutropenia-preventive agent to address the delayed onset of action of G-CSF in the first week of the chemotherapy cycle.29 These studies identify the potential for additional approaches to prevent febrile neutropenia in the first week of the chemotherapy cycle.
Another potential application of G-CSF biosimilars as a primary prophylaxis was for sacituzumab govitecan (SG)-induced neutropenia and febrile neutropenia.30 The SG label recommends G-CSF support only in response to severe neutropenia, rather than prophylactic use. However, a retrospective study of patients receiving SG for the treatment of metastatic triple-negative breast cancer (where 81% of patients developed SG-induced neutropenia), presented at SABCS 2022, found that patients who received G-CSF underwent more cycles of SG treatment (median five versus four).31 This study identifies the potential application of G-CSF biosimilars for other neutropenia-induced instances, warranting future investigation.
Finally, the role of G-CSF to manage neutropenia induced by other agents besides chemotherapy warrants consideration. A new Delphi consensus amongst a French multidisciplinary steering committee considered the use of G-CSFs to treat neutropenia induced by poly (ADP-ribose) polymerase inhibitors, which are currently used in ovarian and breast cancer therapies.32 The committee did not recommend the use of G-CSFs for this indication at the present time, due to the lack of efficacy data.32 However, further research into this application may be warranted.
Such clinical trials indicate the potential direction of future G-CSF prophylactic approaches, and these recent highlights give an indication of the potential application of G-CSF biosimilars in future prophylactic therapy.
CONCLUSION
Given the importance of G-CSFs in providing supportive care and primary prophylaxis treatment to patients with breast, colorectal, and gynaecological cancers, the use of G-CSF biosimilars should be considered as a cost-effective means of supportive cancer care for the effective prevention of febrile neutropenia. Practitioners should consider the interchangeability of biosimilars for filgrastim and pegfilgrastim to replace reference products as an equally efficacious primary prophylaxis to chemotherapy-induced febrile neutropenia, to decrease overall healthcare cost, and improve patient access to supportive cancer care therapies.








