Abstract
People with migraine, and individuals with other neurological conditions, have suffered in multiple aspects during the COVID-19 pandemic. This paper will discuss the factors that impacted the neurology department. The emergence of severe acute respiratory syndrome coronavirus 2 in late 2019 has generated new challenges in healthcare systems across the globe. Similar to the fields of pulmonology and cardiology that saw an increase in research, the neurology department was in search of possible relationships between COVID-19 and other medical conditions. Research on the possible common pathophysiological mechanism between COVID-19 and migraine is currently being studied. The most recent hypothesis suggests the following: migraine is caused by an increased release of calcitonin gene-related peptide from the trigeminal ganglion, which will cause an increase in nitric oxide and IL-1β, resulting in vasodilation and inducing hyperalgesia. COVID-19 causes an increase in nod-like receptor protein 3, which causes the production of IL-1β and again induces an inflammatory response. This review article looks at the mechanisms of migraine and COVID-19, and tries to link a common pathophysiological pathway between the two. This report also serves as a gateway for further research regarding possible management that could potentially target both of these mechanisms.
Key Points
1. The authors examine existing literature to investigate possible pathologies between migraine and COVID-19 infection, endeavouring to establish whether there is a common physiological pathway between the two.2. The authors discuss the differences between COVID-19-associated headaches, which are holocranial and pressing, and the classical presentation of migraine.
3. This article also examines the impact upon the treatment of migraine during the height of the COVID-19 pandemic, and the effects of external stressors on patients.
INTRODUCTION
Migraine is a complex, neurovascular, inflammatory condition defined as “an intense pulsing or throbbing pain in one area of the head” that could be chronic or episodic, according to the definition of the National Institute of Neurological Disorders and Stroke (NINDS).1
The emergence of COVID-19 has raised the worries of physicians across all specialties, including neurologists, with the main concern being the protection of vulnerable patients and tracking of possible neurological changes caused by the disease.2 COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. SARS-CoV-2 belongs to the family of coronaviruses, the same viral family as acute respiratory distress syndrome and Middle East respiratory syndrome.3 Coronavirus is an enveloped, single-stranded RNA virus composed of several proteins: spike (S), membrane, envelope (E), and nucleocapsid proteins.4,5 The protein S is responsible for viral entry into the host cell;5 the membrane protein is essential for the formation of the viral shape;6 the protein E is responsible for several processes in the host cell such as inhibition of host cell stress response, ion channels activity, and mature virion secretion from the host cell;4,7 and, finally, the nucleocapsid protein assists in the packaging of genomic RNA.8
Although migraine and COVID-19 do not share risk factors, they have multiple comorbid diseases such as hypertension and cardiovascular and allergic diseases.2,9,10 Contrary to migraine, which is more prevalent in younger females, there appears to be a higher risk of more severe SARS-CoV-2 infection in older males.2,9 This paper focuses on the correlation of migraine and COVID-19 in regard to their mechanism of action and pathophysiology. It also provides a summary of possible preventative measures to migraine and COVID-19 that may serve as a path for clinicians and doctors to conduct more research testing the credibility of those inferences.
Migraine in the Era of COVID-19
The phenotype of COVID-19 headache in people with migraine was described as holocranial and pressing, and may be associated with phonophobia, or less often photophobia, nausea, and vomiting. These features are different from the classical presentation of migraine.2,11,12 Moreover, these headaches do not respond to abortive migraine drugs such as fremanezumab and sumatriptan.13,14 Headache manifestations in patients with COVID-19 may present as secondary, or exacerbations of primary headache, and it is vital for neurologists to identify the correct diagnosis for proper treatment.13
Several studies done during the COVID-19 pandemic on people with migraine have shown that the overall condition of the pain was improved in the majority of the participants.15-18 Research was conducted to study the impact of the COVID-19 pandemic on people with migraine based on the headache frequency, intensity, and medication consumption during quarantine. The results showed that almost half of the participants had a decrease in migraine frequency, nearly one-quarter did not experience any change in their condition, while less than one-third reported worsening of their migraine.15 Factors that were associated with the improvement of migraine during quarantine ranged from social and familial support, owning outdoor living space, a full-time job, maintaining a positive mindset during quarantine, an increase in leisure activity time, and many more that reduced the surrounding stressors.15
As a matter of fact, according to a study conducted in a paediatric migraine centre in Italy, there was an evident reduction in intensity and frequency of migraine attacks in children and adolescents during the pandemic, mainly due to the psychosomatic nature of the disease in this age group.18 However, the opposite serves true regarding factors that worsened migraine during the pandemic. Examples include stressors related to loss of job or increase in workload (especially for healthcare providers), being single, social isolation, anxiety and depression, and lack of physical activity.15,17 People with migraine who were infected with SARS-CoV-2 reported worsening of their conditions, either by an increase in migraine frequency or an increase in the intensity of the pain.15,19 The increase in migraine frequency was postulated to be due to fever, sleep disturbances, and dehydration that was accompanied by acute COVID-19 infection.2 However, is the worsening of migraine headache attributed only to the stressors that are associated with the disease, or due to a potential common pathway that is shared between the two diseases? In this article, the authors will try to understand the pathophysiology of migraine and COVID-19 and correlate as to why people with migraine saw worsening of their attacks.
METHODS
The authors utilised PubMed as the main database to gather the most accurate and updated journal articles. They searched for the following keywords: “COVID-19,” “SARS-CoV-2,” “headache,” ”migraines,” “mechanism of action,” “NLRP3,” “CGRP,” “pathophysiology,” and “management.” The results were an astonishing number of more than 10,000 research articles and journals in total, many of which had little to do with what the authors were looking for. Therefore, they attempted to narrow down the results by combining certain keywords to attain a desired and specific result. The results were placed in order of most relevant search according to PubMed’s display options. The authors only considered articles that were published in English and were open access.
Following this, the authors went through all the articles, removed the duplicates, and excluded the ones that they deemed irrelevant to their report. As the authors were reviewing the articles, they included those that had information relevant to the main purpose of their article. When reading many of the articles regarding the mechanism of migraine, the authors noticed a trend in almost all of them, mainly that the majority referenced two old research articles that described one of the original postulated pathophysiologies of migraine. These articles go back as far as 1961 and 1972. Therefore, the authors used these two journals as a credible reference for the exact pathophysiology of migraine. Furthermore, when the authors were looking through the journals that described the mechanism of COVID-19, they ran into a similar situation where some articles were referencing older journal articles that describe the pathophysiology of coronaviruses in general. Thus, the authors checked these articles, read them, and included them as credible references to their review.
DISCUSSION
Correlation Between the Mechanisms of Migraine and COVID-19 Headaches
In addition to all the environmental factors, the physiological mechanism of COVID-19 infection is hypothesised to play a major role in the exacerbation of migraine attacks. To understand the correlation between COVID-19 and migraine, it is imperative to first understand the pathogenesis of migraine and COVID-19 independently.
The mechanism behind a migraine attack mainly revolves around cortical spreading depression. This is defined as a slowly transmitting wave of neuronal and glial depolarisation that lasts for a few minutes.20 During this propagating wave, ions, protons, and inflammatory agents are fluxed through the neurons in the cortex, leading to the activation of meningeal nociceptors.20-22 This mechanism is responsible for the aura that precedes migraine and triggers the attack.20 An early reported pathophysiology of migraine is dependent on serotonin, also known as 5-hydroxytryptamine (5-HT).23 A central deficiency of serotonin neurotransmission accompanied by central super sensitivity to 5-HT receptors was one of the first postulated theories for the genesis of migraine.24 Evidently, males tend to synthesise 5-HT at a faster rate compared with females, while females utilise serotonin at a faster rate, hence the higher incidence of migraine in females compared with males.25 Serotonin receptors are highly expressed in the trigeminal vascular system.26 The low level of serotonin induces vasodilation and, for the duration of the attack, an increase in serotonin metabolites levels will be observed in the urine.27,28 In addition, 5-HT reduces calcitonin gene-related peptide (CGRP) levels, which are also thought to be an essential part of migraine pathogenesis.28
CGRP and nitric oxide (NO) are involved in the second possible mechanism of migraine (Figure 1A).9,26 CGRP is a 37 amino acid neuropeptide with two main isoforms: α-CGRP and β-CGRP.29 Both of these isoforms are widely distributed in the peripheral and the central nervous systems (CNS). α-CGRP is predominantly expressed in the somatosensory peripheral nervous system and CNS, while β-CGRP is highly expressed in motor neurons and the enteric nervous system.30 α-CGRPs are thought to play an essential role in migraine pathogenesis as they are overexpressed in the trigeminal ganglion (TG) neurons.31 Inflammatory mediators such as TNF and IL-1β, are responsible for the increase of CGRP in the meningeal vasculature, causing the release of NO, eventually leading to vasodilation.32 One study has shown that the administration of glyceryl trinitrate to healthy individuals will cause an increase in NO, causing migraine-like symptoms.33 Interestingly enough, NO also induces further secretion of CGRP from TG neurons.34 CGRP that is released from the neuronal cell body within the TG leads to the release of more inflammatory mediators, namely IL-1β, which is responsible for the neurogenic inflammation, causing a vicious cycle of CGRP release and inflammatory loop.32 The accumulation of CGRP in the trigeminal nucleus caudalis excites secondary neurons leading to central sensitisation, and the manifestation of hyperalgesia and allodynia that are seen in people with migraine.32
The third proposed mechanism of migraine pathophysiology is associated with the nod-like receptor protein 3 (NLRP3) inflammasome complex (Figure 1B). Inflammasomes are cytosolic protein complexes that activate caspase-1 to secrete proinflammatory cytokines, namely IL-1β and IL-18.35,36 NLRP3 inflammasomes are found in TG neurons, microglia, oligodendrocytes, astrocytes, and neurons.10 One study conducted on mice suggested that glyceryl trinitrate increased the formation of NLRP3, thus further promoting the release of IL-1β.37 To further understand the association between NLRP3 and migraine, the researchers gave the affected mice NLRP3 or IL-1β inhibitors. The results were surprising, as NLRP3 and IL-1β inhibitors reduced CGRP levels, and migraines resolved in the mice.37 In addition, the increase of NO results in the overexpression of NLRP3 inflammasomes, leading to the production of proinflammatory cytokines such as ILs, prostaglandins, leukotrienes, and TNF, which all amplify the inflammation.9,32 In fact, new research is proposing another perspective of migraine pathophysiology that depicts the involvement of NLRP3 inflammasomes.10 Triggers of migraines overexcite the brain, resulting in cortical spreading depression. As mentioned previously, this induces potassium efflux out of the neuronal cell, which causes additional stress to the cell.10 This cellular stress promotes the formation of reactive O2 species (ROS). Following a complex inflammatory process, NLRP3 is formed and activates IL-1β. This cascade continues until it reaches the trigeminovascular system and aggravates it, triggering the CGRP pathway.10
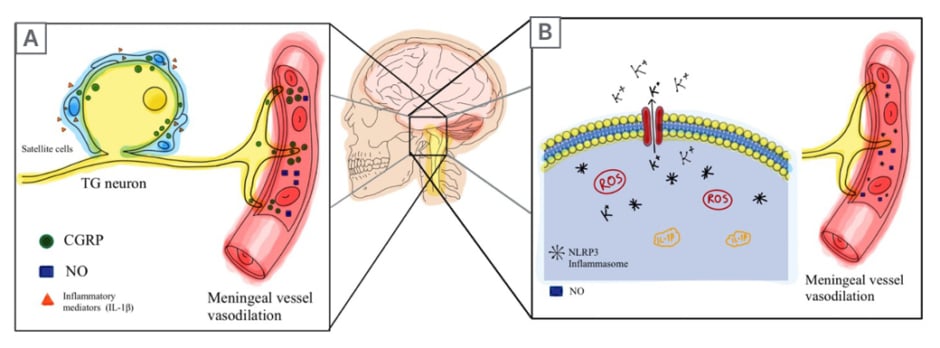
Figure 1: Calcitonin gene-related peptide and nod-like receptor protein 3 inflammasome-related migraine pathways.
A) An initial inflammatory reaction occurs that activates the TG. This induces the release of CGRP into the meningeal vasculature, leading to the release of NO in the vessels, promoting vasodilation. Additionally, CGRP is released from the TG neuron cell, stimulating the surrounding satellite glial cells. Consequently, more inflammatory mediators such as IL-1β and IL-6 are released and activate the TG (causing an inflammatory vicious cycle).
B) Triggers of migraines (sleep deprivation, hunger, etc.) or genetic factors induce CSD. K+ ions are effluxed outside of the cell due to CSD, inducing cellular stress. ROS are formed either via the mitochondria, or other pathways, in response to stress. The imbalance of ions along with the newly formed ROS causes the assembly of NLRP3. Once NLRP3 is formed, caspase-1 is activated and pro-IL-1β and pro-IL-8 are turned into their active forms. These mediators induce a signalling-neurogenic inflammation in the neighboring cells. Then, the trigeminovascular system is activated and the CGRP pathway is initiated.
CGRP: calcitonin gene-related peptide; CSD: cortical spreading depression; K+: potassium; NLRP3: nod-like receptor protein 3; NO: nitric oxide; ROS: reactive O2 species; TG: trigeminal ganglion.
Another concept that sheds light on NLRP3 involvement in migraine is the fact that a mutation in the NLRP3 gene causes the autosomal dominant disease known as cryopyrin-associated periodic fever syndrome.36,38 Mutation in this gene instigates high production of IL-1β and systemic inflammation, leading to migraine-like attacks.10,38 Hence, NLRP3 indirectly induces migraine attacks via the production of IL-1β.
Although the pathophysiology of COVID-19 is not yet clearly understood, there are some theories about what might cause the disease. It is believed that the binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE-2) receptors in the upper respiratory tract provides viral entry access to the cytosol.9,39 In fact, SARS-CoV-2 has multiple entry access points into the CNS: either retrogradely via ACE-2 receptors in the trigeminal nerve terminals of the nasal cavity, through the cribriform plate of the ethmoid, or the circulatory system leading to cerebral circulation in the late stages of the disease.40,41 Several studies suggest that the pathophysiology behind COVID-19 is related to a cytokine storm.5,10,42-44 SARS-CoV-2 induces a hyperinflammatory state through the recruitment of IL-6, which dysregulates other inflammatory and coagulation factors.45 Therefore, it is hypothesised that during COVID-19 infection, there is a meningeal peripheral sensitisation that activates the TG, causing a headache that resembles migraine.43 The cytokine storm may also incite blood–brain barrier instability, which activates the trigeminovascular system, causing headaches.45
Another suggested pathophysiology of COVID-19 is related to NLRP3 inflammasomes.10 The most important protein that might be responsible for the host cell inflammatory cascade is the protein E. Hence, the hypothesised inflammation pathogenesis of COVID-19 is as follows: S glycoprotein attaches to the ACE-2 receptors in the upper respiratory tract.46,47 As ACE activities are disturbed, angiotensin (Ang) II will not be converted to Ang I, resulting in an accumulation of Ang II. Elevated levels of Ang II lead to the activation of NLRP3, as well as the release of CGRP into the meningeal vessels, causing vasodilation and migraine-like headache symptoms.9,48 Moreover, once the virus enters the host cell, a cascade of inflammatory reactions is triggered, which involves the release of NLRP3 inflammasome and nuclear factor-κB (Figure 2A).9,39 NLRP3 inflammasomes then initiate the cytokine storm.49
The inflammatory reaction is believed to be mediated by the E viroporin. Studies have shown that the E viroporin of SARS-CoV-2, which causes acute respiratory distress syndrome, activates NLRP3 inflammasomes in the host.3,5 NLRP3 inflammasome is activated through an imbalance of calcium (Ca2+) cations within the cell due to the formation of Ca2+ channels by the protein E of SARS-CoV-2.5 This imbalance causes cellular stress and the formation of mitochondrial ROS that induce the assembly of NLRP3.10 Although it is not yet confirmed whether a similar viroporin is present in SARS-CoV-2, it is suspected that such a mechanism is the cause of the inflammatory reaction of COVID-19; however, further studies need to be done.5,10 Another possible factor that might activate NLRP3 inflammasomes during COVID-19 infection is the hyperactivation of P2X7 receptors due to the elevated levels of extracellular adenosine triphosphate.44 P2X7 receptors are cation channels that are highly expressed in multiple parts of the CNS and are activated by adenosine triphosphate.42 Once NLRP3 inflammasomes are activated, the same mechanism as migraine follows, in which caspase-1 is activated and cleaves the inactive pro-IL-1β into its active form IL-1β.5 Eventually, IL-1β triggers a cascade of subsequent mediator activation such as IL-6, TNF, leukotrienes, and prostaglandins.5
The third mechanism of SARS-CoV-2 infection involves the CGRP pathway and it is currently under study.11 As the virus gains entry via ACE-2 receptors, an inflammatory reaction is initiated in the nasal mucosa, which involves an extreme increase in TNF-α.50 TNF-α further promotes a nociceptive process that increases the expression of IL-1β in the nasal mucosa.51 Both of these mediators, TNF-α and IL-1β, stimulate the release of CGRP from the TG neurons (Figure 2B).11 Simultaneously, CGRP promotes the release of cytokines such as IL-1β from the TG satellite glial cells.11 IL-6 and Ang II are also thought to induce a systemic increase in CGRP, not only from the TG.48 In fact, the presence of CGRP in small- and medium-sized sensory neurons as well as in the TG activates the trigeminal nerve endings, inducing a similar mechanism as migraine.52
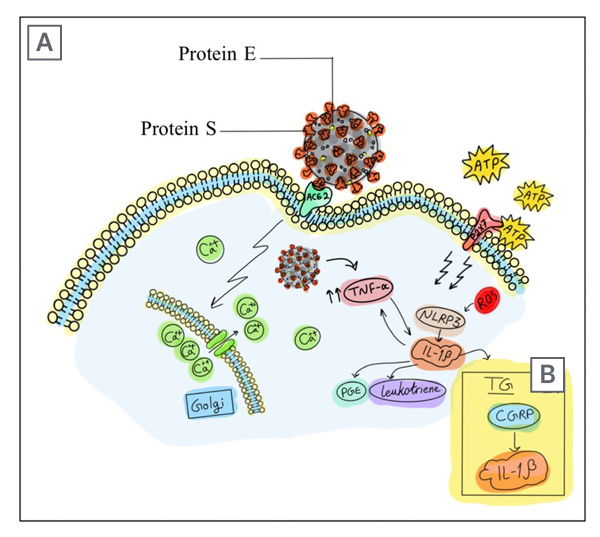
Figure 2: Severe acute respiratory syndrome coronavirus 2 spike protein provides access into the cell through angiotensin-converting enzyme 2 receptors.
A) Viroporin E is believed to start an inflammatory cascade by forming Ca2+ channels on the Golgi apparatus membrane.. The imbalance of intracellular Ca2+ causes the formation of mitochondrial ROS. In addition, the cellular inflammatory state leads to elevated levels of ATP, which activates P2X7 receptors. The ion imbalance that is caused by E protein Ca2+ channels and the activation of P2X7 receptors promote the formation of NLRP3 inflammasome complex, which provokes COVID-19 headache.
B) The other mechanism suggests that the recruitment of TNF-α, in response to viroporin E entry, increases the expression of IL-1β. This will cause the release of CGRP in the TG, which triggers a similar mechanism to a migraine attack. The released CGRP stimulates the surrounding satellite glial cells to release more inflammatory cytokines.
ACE-2: angiotensin-converting enzyme 2; ATP: adenosine triphosphate; Ca2+: calcium; CGRP: calcitonin gene-related peptide; E: envelope; NLRP3: nod-like receptor protein 3; PGE: prostaglandin; ROS: reactive O2 species; S: spike; TG: trigeminal ganglion.
A tangential mechanism for COVID-19 headache may be due to hypoxaemia, in which the alveolar damage and lung oedema reduce O2 levels in the CNS. An acidic environment is established following hypoxia-induced anaerobic metabolism, leading to cerebral vasodilation and cerebral oedema, hence the headache.53
Considering all the mechanisms mentioned above, the authors can correlate the common pathophysiology of COVID-19 and migraine. COVID-19 increases the release of NLRP3, which causes the release of inflammatory mediators (especially IL-1β and NO). Migraine attacks are provoked by CGRP, which causes an inflammatory mediator release cascade (including IL-1β and NO). In both migraine and COVID-19, mitochondrial ROS are formed in a similar manner, eventually creating an almost identical further molecular inflammatory pathway that involves NLRP3 inflammasome.10 Not only is CGRP released in the nasal mucosa following the increase in TNF-α during COVID-19 infection, but it is also released from the dura mater, thus increasing the meningeal blood flow.11,54 Therefore, the cytokines will induce a secondary activation of meningeal nociceptors, causing headache.11
A CGRP-induced headache caused by COVID-19 may resemble migraine due to the common pathophysiology of CGRP. In fact, it was found that patients with COVID-19 who had an early diagnosis of migraine had longer and more intense COVID-19-associated headaches.55 This phenomenon is explained by the increased nociceptive processing in the trigeminal cervical complex after COVID-19 infection.55 The peripheral sensitisation during COVID-19, in patients with sustained migraine attacks, may induce central sensitisation and reduced pain threshold, leading to prolonged and exaggerated response.55 Though the results are remarkable, more research needs to be done to confirm the hypothesis that COVID-19 may cause a more severe headache in people with migraine.
Management of Migraine During COVID-19
According to various sources, the recommended treatment for migraine ranges from nonsteroidal anti-inflammatory drugs (NSAID) to renin-angiotensin system (RAS) inhibitors. It all depends on the severity and whether the attacks are chronic or acute. Deciding on which migraine medication to use during COVID-19 is crucial in determining whether the course of the headache will improve or worsen, according to the previously discussed mechanisms. This section will focus on the management of migraines and the implemented changes that took place due to the pandemic. According to the American Headache Society (AHS), NSAIDs and triptans are the first-line treatments for acute migraine attacks, and this was backed up by strong evidence from research.56 Other preventative medications include onabotulinumtoxinA injections, CGRP monoclonal antibodies, β-blockers, and angiotensin receptor blockers.44,56
With the pandemic and lockdown, research was conducted to study the possible link and interaction between COVID-19 and these medications. One initial fear of the use of NSAIDs and RAS inhibitors was due to the belief that they may result in worse COVID-19 clinical symptoms, due to proven studies that RAS blockers up-regulate ACE-2 expression.56,57 Since the evidence is lacking and no proper link has been established between COVID-19 and these medications, the choice to use them remains a patient-to-doctor discussion.
CGRP monoclonal antibodies are commonly used as a prophylactic for migraine attacks. As of now, neurologists are recommended to keep prescribing anti-CGRP monoclonal antibodies since there is no evidence suggesting increased susceptibility to SARS-CoV-2 infection.29 Since CGRP plays a significant role in the pathogenesis of COVID-19, clinical trials, namely BHV3500, are being conducted to evaluate the efficacy of CGRP-antagonists in the treatment of SARS-CoV-2 infection.58 The trial raises suspicion that monoclonal antibodies against CGRP would be the preferred choice of treatment for people with migraine who are infected with COVID-19. Other possible medical interventions for COVID-19 headache and migraines are blockade of IL-6, as it is linked to increased severity of the infection. Furthermore, drugs targeting the NLRP3 inflammasome pathway are a potential treatment for migraine and COVID-19, some of which are currently under clinical development.5,10,59,60
CONCLUSION
To sum it up, people with migraine who were not infected with SARS-CoV-2 had an overall improvement in their condition during quarantine due to a decrease in the surrounding stressors, whereas people with migraine infected with COVID-19 reported a worsening of their condition. Multiple environmental and pathophysiological factors were considered to reach this conclusion. Up to this point, research has attributed this trend to be caused by the life stressors that surfaced during the pandemic, or to the accompanying COVID-19 symptoms. However, the authors believe that pathophysiological factors, including neuropeptide CGRP and NLRP3, are the cause of the increased severity of migraine attacks in patients with COVID-19.
The overall conclusion of all these studies is summarised by the fact that migraine attacks are caused by an increase in CGRP, NO, and IL-1β. COVID-19 also seems to affect these factors by increasing the stimulation of NLRP3 and possibly CGRP, thus IL-1β and NO are overproduced. This raises the question of whether people with migraine will have an increased risk of frequent and intense attacks when infected with SARS-CoV-2. It also provides a linking point, which makes it easier to conduct further research for a possible drug that targets the potential common pathway. More research needs to be done to fully understand the concept and the correlation between NLRP3 release as a result of COVID-19 and levels of CGRP.







