Summary
Hospitalisation rates for acute bacterial skin and skin structure infection (ABSSSI) are rising and represent a large pharmacoeconomic burden as treatment may involve an extended number of days of antibiotic therapy. This article first aims to provide a review of treatment challenges associated with ABSSSIs in both hospital and outpatient settings, and shows that while more traditionally treatment has been conducted in a hospital setting, for a number of patients, a variety of considerations, including pharmacoeconomics, infection control, and patient preference, has led to the development of recommendations to assess the eligibility of patients for early discharge from hospital to complete their antibiotic regimen in the outpatient setting. However, such patients require monitoring for drug adherence to oral regimens or complications associated with daily intravenous administration, such as injection site reactions and infection. This review also focuses on one of a number of new antibiotics for ABSSSI, dalbavancin, as the long-acting glycopeptide with the most clinical experience to date. This antibiotic has been shown to be as effective as a daily/twice daily regimen with similar safety profiles. Health economic analysis of dalbavancin is also presented. It has shown that in some, though not all, clinical settings a reduction in the overall treatment cost is evident as, despite a higher medication cost, the lower hospitalisation time can lead to greater cost savings. In conclusion, while the burden of ABSSSI is rising, new treatment options provide additional therapeutic choice, although pharmacoeconomic considerations might limit use in some cases.
INTRODUCTION
ABSSSIs include cellulitis/erysipelas, wound infections, and major cutaneous abscesses. They are defined by lesion size (≥75 cm2), and are demarcated by area of redness, oedema, or induration.1 An ABSSSI is considered ‘complicated’ when surgery is required in addition to antibiotic treatment and/or the infection reaches deeper subcutaneous tissue.2 Around a third of ABSSSI are caused by methicillin sensitive (MSSA) or methicillin resistant Staphylococcus aureus (MRSA). Another major cause is Streptococcus species, with fewer ABSSSI being due to Klebsiella and Enterococcus species, Pseudomonas aeruginosa, and Escherichia coli.3,4
Risk factors and comorbidities associated with ABSSSIs include prior episodes; older age; diabetes; intravenous (IV) drug use; cardiovascular disease; chronic wound or ulcer presence; peripheral vascular disease; chronic renal failure; malnutrition; smoking; skin conditions; obesity; and cirrhosis.3,5 The most common risk factors for a complicated ABSSSI are antibiotic use within 30 days and hospitalisation within 6 months.2 Patients with comorbidities and complicated ABSSSI experience higher rates of reinfection or recurrence (9.6% versus 5.2% without comorbidities); longer hospital stays (mean: 19.9 days versus 13.3 days); a longer time to clinical stability (10.4 days versus 7.1 days); and a higher mortality rate (4.0% versus 1.1%).6
This article presents an overview of ABSSSI treatment and the challenges associated with such in a hospital or community setting; provides a checklist to help determine eligibility for early discharge of a patient hospitalised with an ABSSSI; and discusses efficacy, tolerability, and pharmacoeconomic data for one of the new antibiotics for an ABSSSI, dalbavancin, as the long-acting glycopeptide with the most clinical experience to date.
TREATMENT FOR ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTIONS
ABSSSI treatment is directed by clinical manifestation, infectious agent(s), and purulence. Important aspects of clinical care include stabilisation of physiology if sepsis exists; surgical drainage; mechanical or chemical debridement; and antibiotic therapy.3,7,8 Table 1 shows examples of antibiotic options for an ABSSSI. For mild infections, oral formulations are recommended, with IV delivery reserved for moderate–severe infections.10 To mitigate recrudescence, treatment is usually recommended for 7–10 days or longer if symptoms/signs do not adequately resolve.3
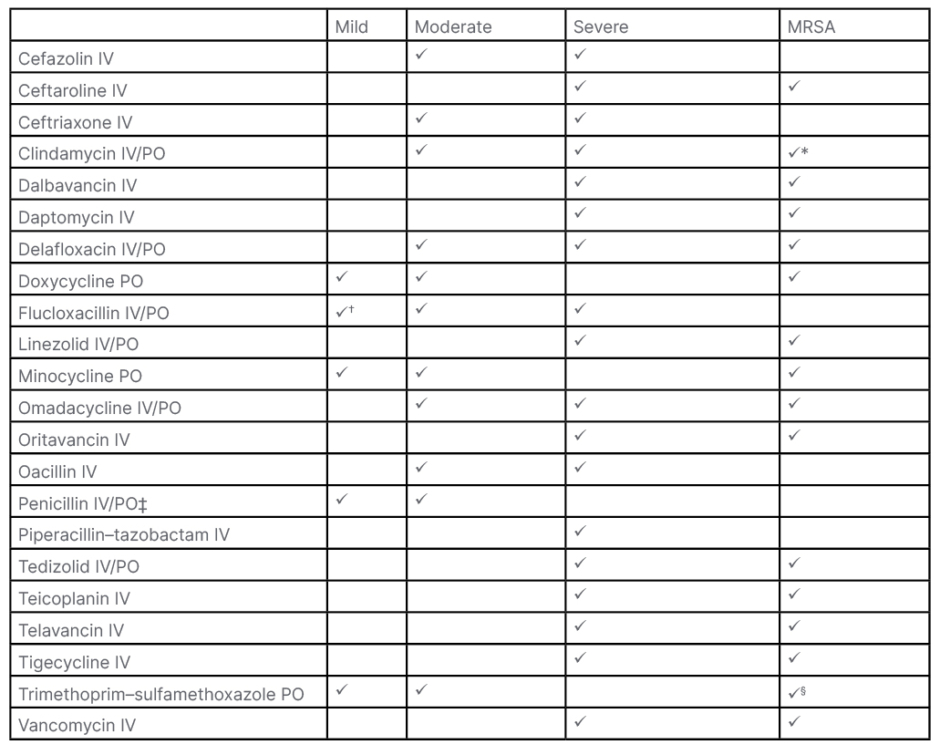
Table 1: Examples of antibiotics prescribed for an acute bacterial skin and skin structure infection.3,7,8,9
*Not always susceptible, especially healthcare-associated strains.
†PO
‡Staphylococci are often resistant.
§Not always susceptible.
IV: intravenous; MRSA: methicillin-resistant Staphylococcus aureus; PO: by mouth.
Antibiotic treatment recommendations differ by indication and country-specific guidelines.7 According to Michael Wilke, Inspiring-health GmbH, Waldmeisterstrasse, Munich, Germany, and Medical School Hamburg, Am Kaiserkai, Germany, and colleagues, choice of therapy for a bacterial disease is a case of “choosing the right antibiotic at the earliest possible moment to maximise the chances of curing the infection […] and minimising risk of developing resistance.”11
KEY CHALLENGES ASSOCIATED WITH TREATING A PERSON WITH AN ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTION AS AN INPATIENT
Hospitalisation for and rates of ABSSSIs are rising.12 Cellulitis was found to have an incidence rate of 24.6/1,000 persons/years in a UK-based study.13 As such, ABSSSIs represent a large pharmacoeconomic burden.14 For example, a study of non-severe ABSSSI examining data from Spain, Italy, and Austria estimated a total annual expenditure of 13.5 million EUR, 9.9 million EUR, and 3.4 million EUR, respectively.15
Issues associated with ABSSSI treatment include hospitalisation and length of stay (LOS). A prospective study of 94 patients presenting to the emergency department found that for 85.1% the primary reason for admission was IV antibiotic need, a major driving factor for LOS.16 While patients may also be admitted to monitor comorbidities, an observational study involving 520 hospitals and 600,000 patients found that 60% of those admitted with an ABSSSI had no significant systemic symptoms or comorbid conditions.17
MRSA-related ABSSSI present an increased cost burden when hospital stay is extended,18 with the need for patient isolation and enhanced healthcare professional protection measures.19 An analysis of German healthcare data found that average LOS for patients with MRSA was 25.8 days (mean cost: 16,024 EUR/stay), compared with 14.6 days for patients without MRSA (mean cost: 8,198 EUR/stay). As costs per day were similar (621 USD versus 561 USD, respectively), the cost difference was predominantly attributed to LOS.19 This may be problematic financially as in many countries, treatment for a named condition is compensated by insurance companies to a set amount based on average cost per patient. This means with an LOS longer than the mean, the hospital/healthcare authority will be responsible for additional costs.11
For the patient, long LOS presents medical, social, and psychological difficulties, such as the chance of picking up another infection,20 or an infection/complication associated with MRSA and vancomycin-resistant enterococci.21,22
Longer hospitalisations are also associated with feelings of isolation, depression, worry about an inability to maintain family responsibilities, and financial problems due to inability to work.20,23 Medically, extended LOS are associated with pressure sores, deep vein thrombosis, and deconditioning, especially in elderly patients who may also experience increased confusion, loss of function, and immobility.20
TREATING ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTIONS IN THE OUTPATIENT SETTING
An economic model developed to compare in- versus outpatient treatment of Gram-positive ABSSSI when using a daily IV antibiotic found that total ABSSSI treatment costs were lowest when patients were treated in the outpatient setting.24 This has led to identification of appropriate patients with ABSSSI eligible for early discharge when home treatment is viable.25 A checklist designed by Wilke and colleagues can be used at the bedside to assess if a person with an ABSSSI could potentially be discharged to home care (Box 1). These criteria also take into account clinical experience of the authors and a meta-analysis which showed that, following assessment of clinical and infection stability, over one-third of hospitalised patients with MRSA could be eligible for early discharge.26

Box 1: Checklist to determine eligibility for early discharge to outpatient antibiotic therapy.26
In some countries, outpatient treatment is formalised as outpatient parenteral antimicrobial therapy (OPAT), which may include a patient self-administering an antibiotic at home or with the help of a carer/healthcare professional, and/or them coming into an outpatient clinic as needed.27 Good practice recommendations for OPAT delivery have been published in the UK.28
Outpatient treatment of an ABSSSI can be via oral or IV antibiotic therapy. However, challenges with home-based treatments include that while oral antibiotics are convenient and as effective as IV preparations when taken correctly,7 non-adherence may occur. A study of linezolid use following hospital discharge found that 24.1% of 1,046 patients did not pick up their prescribed medication from their pharmacy, indicating non-adherence.29 Similarly, a study with people with uncomplicated ABSSSI (n=87) found that while 96% reported full adherence on questionnaires, electronic bottle cap opening data recorded full adherence in only 57%. After 30 days of treatment, 46% had a poor clinical response, associated with lower adherence.30 An alternative to this, with better rates of adherence, is the use of long-acting antibiotics, discussed below.3
One advantage of inpatient treatment is that tests for clinical assessment or therapeutic drug monitoring can be conducted more rapidly. In OPAT, testing is less frequent, and results may take longer to be reported. Other potential problems associated with OPAT are that the patient might need to be responsible for peripherally inserted intravenous device care, and for attending appointments.27,31
Antibiotic treatment-associated complications, such as nephrotoxicity, venous access issues, drug-related adverse events (AE), and skin rash, need to be closely monitored in the outpatient setting.32 One study examining home infusion therapy found AE rate to be 7.7/1,000 OPAT days for vancomycin (n=1,105) and 3.2/1,000 OPAT days for daptomycin (n=83). The rate of antimicrobial interventions was 27.1/1,000 and 5.6/1,000 OPAT days, respectively, with hospital readmissions of 18% for vancomycin and 21% for daptomycin.33
Analysis of National Health Service (NHS) England hospital episode statistics showed that over 20% of people who had been discharged following hospitalisation for cellulitis were readmitted, with over half in the first month. This was estimated to cost 29 million GBP per year.34 Readmission for cellulitis may occur for a variety of reasons, including undertreatment with antibiotics35 and misdiagnosis.36
DALBAVANCIN USE IN ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTIONS: EFFICACY AND SAFETY
Newer drugs approved for ABSSSI include the long-acting antibiotics dalbavancin and oritavancin. Their prolonged half-lives mean that a single infusion can deliver antibiotic efficacy over several days.3 As the long-acting glycopeptide with the most clinical experience to date, the focus here will be on dalbavancin, an intravenously delivered semisynthetic lipoglycopeptide antibiotic with proven potency against MSSA, MRSA, and other ABSSSI-related staphylococci, enterococci, and streptococci.37,38 Resistance to dalbavancin is rare.39 Dalbavancin was approved by the European Medicines Agency (EMA) in 2015 for the treatment of ABSSSI in adults.37,40 Similar lipoglycopeptide antibiotics include teicoplanin and vancomycin,41 although dalbavancin has faster bactericidal activity and a longer half-life than these.41
Due to its long terminal half-life of around 15 days,40-42 dalbavancin is administered either as a single 1500 mg infusion or a 1000 mg infusion on Day 1, followed by a 500 mg infusion on Day 8.37 Clinical success rates and AEs are non-inferior between dosing regimens.43 Another benefit is that IV administration does not require placement of a peripherally inserted intravenous device,41 as may be needed for daily IV administration, thereby decreasing the risk of complications of such.32 The long half-life of dalbavancin may mitigate the risk of recurrence of ABSSSI in some patient groups, in keeping with results of a trial of short course (six versus 12 days) therapy in severe cellulitis.35
Double-blind, non-inferiority trials found clinical response times and percentage of participants with an early clinical response were similar (around 80%) for IV dalbavancin (525/659) and vancomycin±linezolid (521/653) administered IV every 12 hours for ≥3 days, with the option to switch to twice-daily oral linezolid for 10–14 days. Over 90% in each group achieved an early clinical response, and around 97% of patients with MSSA or MRSA achieved a clinical response by the end of treatment.44
Occurrence of any AE (32.8% versus 37.9%, respectively), diarrhoea (0.8% versus 2.5%), and pruritis (0.6% versus 2.3%) was signi ficantly lower with dalbavancin, with therapy discontinuation due to an AE being approximately 2% for both groups. In these trials, the most frequent AE with dalbavancin was nausea (2.5% compared with 2.9% in the vancomycin–linezolid group). A serious AE was reported in 2.6% of the dalbavancin group and 4.0% of the vancomycin–linezolid group (p=0.16). While it may be considered that the extended half-life of dalbavancin could lead to longer time with an AE, these trials showed mean AE duration was similar between groups (8.7 days),44 in keeping with the authors’ clinical experience.
To be a viable option, the efficacy and safety of dalbavancin needs to be proven to be similar to other antibiotic choices. A network meta-analysis (NMA), including 17 studies of which three involved dalbavancin, found similar efficacy and safety for dalbavancin compared to vancomycin, daptomycin, teicoplanin, linezolid, and tigecycline. There was also a similar incidence of AE rates (44.9%) compared with all other antibiotics (46.8%). For all-cause mortality, there was a significantly higher risk with vancomycin, linezolid, and tigecycline versus dalbavancin. For serious AEs, there was a significantly higher risk with vancomycin and daptomycin, and for AE incidence there was a significantly higher risk with linezolid only. However, these results are to be viewed with caution due to the considerable design heterogeneity of included studies.45
Another NMA found dalbavancin had similar efficacy and early clinical response to vancomycin and oritavancin for complicated Gram-positive ABSSSI including MRSA.46 Oritavancin is an IV lipoglycopeptide with a half-life of 10.2 days, administered as a single infusion, used for Gram-positive bacteria including MRSA and vancomycin-resistant enterococci.47 This NMA found fewer overall AEs with dalbavancin compared to standard of care (SoC) (odds ratio: 0.77; 95% confidence interval: 0.64–0.93), although the odds of serious AEs were similar, and there were similar AEs/serious AEs as otrivancin.46
Clinical trials of oritavancin have shown efficacy similar to vancomycin with nausea, headache, vomiting, cellulitis, increased alanine aminotransferase, and infusion site phlebitis being the most frequent AEs. In the oritavancin group, 3.6% discontinued due to a treatment-emergent adverse event, namely cellulitis, infection, or osteomyelitis.48 Caution is advised if oritavancin is administered with drugs with a narrow therapeutic window predominantly metabolised by an affected cytochrome P450 enzyme, as concentrations increases or decreases may occur. As oritavancin binds to or prevents the action of phospholipid reagents that activate coagulation, blood concentrations of oritavancin following a 1200 mg dose may lead to falsely elevated results in some laboratory coagulation tests.49
Following formal clinical trials, several studies have investigated dalbavancin efficacy and safety in real-life settings. In an Austrian cohort, 26 patients treated for a Gram-positive ABSSSI showed a clinical cure rate of 77%, with 23% of patients switching to a different antibiotic.50 An similar clinical cure rate (75%) was found in an Italian study (n=170), where dalbavancin was found to be tolerable with AEs occurring in 5.4% of patients and one occurrence of Stevens–Johnson syndrome.51
However, in a large retrospective study in the USA (n=418) where patients received either dalbavancin or SoC with another antibiotic, overall treatment success rate was significantly higher with SoC (85% versus 74%; p=0.004). There were also lower 30-day ABSSSI-related readmissions overall (SoC: 14.83%; dalbavancin: 26.32%; p<0.01), although dalbavancin had a better odds ratio for readmission in treatment naïve patients who had not received antibiotics over the past 30 days (0.4; 95% confidence interval: 0.2–0.9).52
An Austrian retrospective, multicentre study of 101 patients (10.9% with an ABSSSI) found that reasons for dalbavancin use included its long half-life; previous antibiotic failure, allergic reaction, or AE; pathogen resistance to other antibiotics; or patient non-compliance. Investigation of 90-day outcomes found that 89% of 94 patients had no evidence of infection, with treatment failure in 5%.53 An observational, retrospective study in 29 Spanish hospitals (n=69; 21.7% with an ABSSSI) found that most patients (97.1%) received prior antibiotic therapy before receiving dalbavancin. Switching to dalbavancin was most often carried out for antibiotic administration ease (73.9%); previous treatment failure (30.4%); antimicrobial resistance (18.8%); and β-lactam allergy (14.5%).54 Finally, in a USA study, the main reasons documented for dalbavancin use over other IV antibiotics was a history of IV drug use; contraindications to other antibiotics; lack of outpatient infusion options; and history of non-adherence to outpatient IV antibiotics.55 In selected patients therefore, dalbavancin appears to be a useful addition to the ABSSSI therapeutic armamentarium.
DALBAVANCIN USE IN ACUTE BACTERIAL SKIN AND SKIN STRUCTURE INFECTIONS: ADHERENCE AND CONVENIENCE
Recurrent ABSSSI are prevalent in people who inject drugs (PWID)56 where medication adherence may be a concern.57 As such, it is postulated that the single-dose regimen of dalbavancin may help optimise treatment adherence.37,58 A retrospective observational study found that even with dalbavancin administration, only 53% of 32 patients, of whom 88% were PWID, completed the intended therapy course, with 31% lost to follow up.59 However, it also found that, compared to PWID discharged into an outpatient antibiotic program prior to dalbavancin use, patients treated with dalbavancin had significantly improved outcomes including shorter hospital LOS, higher clinical cure rates, and lower readmission rates.60
Incidence61 and hospitalisation rates for people with an ABSSSI are highest in those ≥65 years, and LOS is on average longer.12,14 Prolonged hospitalisations for such patients has been associated with complications including immobility, deconditioning, pressure sores, deep vein thrombosis, and nosocomial infections.20 As such, this is another patient population that could benefit from the use of long-acting antibiotic therapy, as it can provide a reduction in LOS in clinically stable patients over 65 years old who do not need to be in the hospital for another reason.18
A further advantage of dalbavancin is to quality of life. A post hoc analysis comparing the two dosing regimens of dalbavancin found that those treated as outpatients (n=386) compared to inpatients (n=312) were more satisfied with their therapy with regard to treatment and care received, effect of treatment, and treatment location. A significantly larger percentage (p<0.001) found outpatient therapy interfered less with their daily activities. However, while a similar percentage in each group were ‘unconcerned’ about their antibiotic treatment (43% versus 44%, respectively), 28% of outpatients versus 6% of inpatients answered the question ‘How often were you concerned about receiving your antibiotic treatment?’ with the choices ‘most/all of the time’ (p<0.001), suggesting the need to optimise patient education and reassurance when treated as an outpatient.62
DALBAVANCIN AND PHARMACOECONOMICS
Although there are many advantages of dalbavancin, drug cost may limit its use in some settings. However, as discussed previously, LOS for people hospitalised with an ABSSSI is paramount in pharmacoeconomic considerations. In the ENHANCE trial, dalbavancin use (n=43) reduced LOS by almost 2 days when compared with SoC.63 Another study utilised French registry data of dalbavancin administration (n=154) for a range of infections including ABSSSI. Here, LOS was found to be almost always shorter in patients who received dalbavancin compared with SoC, by up to 13 days. Cost savings particularly occurred in patients where dalbavancin was used early in treatment.64
With this in mind, it is proposed that reductions to LOS could lead to cost savings that outweigh the price of using dalbavancin. Wilke and colleagues devised a model to determine the economic effects of a single dose of dalbavancin in hospitalised patients in Germany. In those with an ABSSSI, MRSA presence was associated with an increased LOS of 6.45 days and an excess cost of 5,145 EUR per patient. The use of dalbavancin combined with early discharge was projected to lead to average savings of 2,964 EUR.65
A number of studies have examined actual costs and LOS. A pharmacoeconomic study utilising data from Italy, Spain, and Romania (involving around 30,000 patients) estimated a LOS reduction of 2.50–4.15 days with dalbavancin compared with SoC. In the Italian cohort, while drug cost was 37.0% more with dalbavancin, it was offset by a 38.5% decrease in other treatment resources, accounting for slightly lower costs compared to SoC. However, in Romania and Spain, treatment brought a slight increase in cost with dalbavancin use, by 0.1% and 1.0% respectively, compared with SoC.66 In a study examining data from Italy, Spain, and Austria, LOS/1,000 patients hospitalised with a non-severe ABSSSI was reduced by 601.8–782.6 days following dalbavancin use. This led to estimated cost savings of 370,269 EUR–1.1 million EUR.15 Analysis of data from 17 patients with ABSSSIs treated with dalbavancin in a Scottish hospital outpatient setting found that cost of 14 days’ hospitalisation, not including treatment, would have been 53,000 GBP.67
A number of USA studies have all found comparable results, with one showing that LOS was 4.3 days with dalbavancin (n=44) compared with 8.0 days for a SoC comparator group (n=945; p<0.001) and 5.0 days for the national average. Mean total direct cost per person was 2,889 USD for the dalbavancin group compared with 7,863 USD for the comparator group, showing considerable cost savings.68 Another study (n=37) showed mean cost reductions of 40,414 USD/patient and a total of 617 hospital days saved.55 Finally, a meta-analysis using USA healthcare costings found that in comparison to inpatient costs using SoC, outpatient use of dalbavancin was equivalent or lower when hospitalisation costs (6.5–10 days of treatment) were taken into consideration.46 This reduction in hospital days is significant when considering the risks associated with extended hospital stays, especially during the COVID-19 pandemic. Of note though, in another USA study, where the primary outcome was average net cost of care to the healthcare system per patient, calculated as the difference between reimbursement payments and the total cost to provide care to the patient, dalbavancin-related healthcare costs were significantly higher than SoC (4,804 USD±2,271 USD versus 2,709 USD±5,281 USD; p<0.01).52
CONCLUSION
ABSSSI treatment may necessitate an extended hospital stay. Taken as a whole, the studies discussed above demonstrate that judicious use of dalbavancin in selected patient groups could lead to enhanced adherence and overall cost savings when administrated to patients who, according to Box 1 criteria, are eligible for early hospital discharge to continue their outpatient antibiotic therapy. While the overall healthcare economy cost benefit of dalbavancin is likely marginal, and is largely dependent upon the local drug price, there are clearly populations of patients who would benefit from this novel form of therapy. Dalbavancin may allow a decrease in LOS or early discharge, and should be anticipated as opposed to being dedicated to compassionate use after multiple prior courses of treatment. Further research is required to clarify its clinical- and cost-effectiveness versus β-lactam antibiotics, which remain the mainstay of outpatient and inpatient ABSSSI therapeutics in areas with low MRSA endemicity.
Job Code: ADV/MISC/PM/0061;
Date of Preparation: August 2022








