Abstract
Liver transplant is the treatment available for eligible patients with end-stage liver cirrhosis. Acute kidney injury and electrolyte abnormalities are associated with liver disease and can be exacerbated by surgery. Intraoperative renal replacement therapy has been tried in some large centres. The authors discuss the physiological changes and complications during liver transplant surgery, and review literature on the safety, feasibility, benefits, and drawbacks of intraoperative renal replacement therapy during liver transplant surgery.
Key Points
1. Renal insufficiency in liver cirrhosis portends a poor prognosis. Liver transplant is the only treatment for end-stage liver disease, and the surgery is complex, with major disturbances in acid-base and electrolyte status.
2. Intraoperative renal replacement therapy can be an option to optimise acid-base and electrolyte status during liver transplant.
3. This review discusses the limited data of intraoperative renal replacement therapy during liver transplant, and reviews the challenges, benefits, and adverse events described.
INTRODUCTION
End-stage liver disease (ESLD) represents a non-reversible, advanced stage of cirrhosis, where the hepatic architecture is distorted and nodular. It is one of the top 10 causes of death in the USA, and common causes include alcohol abuse and viral infections.
The complications in patients with acute fulminant hepatic failure and ESLD range from bleeding, infections, and malnutrition, to multiorgan failure. Acute kidney injury (AKI) is common (50–58%) in hospitalised patients with cirrhosis.1 It can be precipitated by gastrointestinal bleeding, angiotensin-converting enzyme inhibitors, large volume paracentesis, and infection. Types of AKI include acute tubular necrosis, glomerular pathologies (cryoglobulinaemia, secondary IgA, etc.), and hepatorenal syndrome (HRS). AKI portends a poor prognosis, with one analysis showing a combined mortality rate of 67%, with 1-month mortality at 58%, and 54–79% at 1 year.2
ESLD is a condition that is increasing in prevalence, and has very high morbidity and mortality. Even without complications, the median survival is approximately 2 years.3 Liver transplantation (LT) remains the best treatment option for eligible patients. AKI before LT worsens the prognosis (50% mortality).4 The need for renal replacement therapy (RRT) pre- or post-LT is associated with more than a two-fold increase in risk of death.5 For those undergoing LT, AKI is present in 15–20% on admission, and this number can increase as they wait for LT.6
Eligible patients with ESLD who are dialysis dependent beyond 8 weeks (about 2 months) are considered for simultaneous liver-kidney transplant.7 The pre-, intra-, and postoperative management of LT surgery is challenging, and requires close monitoring of electrolytes, volume status, and acid-base homeostasis.
A recent article6 reviewed the physiological changes in liver cirrhosis, and how intraoperative RRT could impact this. This article does denote the limitations in data, with limited to no controls in available literature. The authors’ review further expands on this, and also analyses data from multiple studies regarding the safety, feasibility, and practical challenges of intraoperative RRT.
This is important, as LT surgery is associated with high morbidity and mortality, some of which are related to complications from AKI and electrolyte disturbances.
INDICATIONS FOR RENAL REPLACEMENT THERAPY IN HEPATORENAL SYNDROME
Dialysis is offered to patients with HRS who are candidates for LT.8 The indications to start RRT are like the general indications for RRT. HRS needing dialysis is associated with a poor prognosis.9 RRT can be a bridge to transplant in those on the waitlist, and those undergoing evaluation for eligibility.
Given the complex physiological changes during LT surgery, some centres are utilising RRT perioperatively (including intraoperatively) in patients with kidney disease.
At present, there is no clear consensus or guideline regarding dialysis initiation.
Continuous renal replacement (CRRT) is the modality that is mostly used.
Entwined Organ Failure and Physiological Changes in Cirrhosis
An understanding of these physiological changes helps us better understand the complications that can arise intraoperatively, and the potential role of RRT in mitigating thesecomplications (Figure 1).
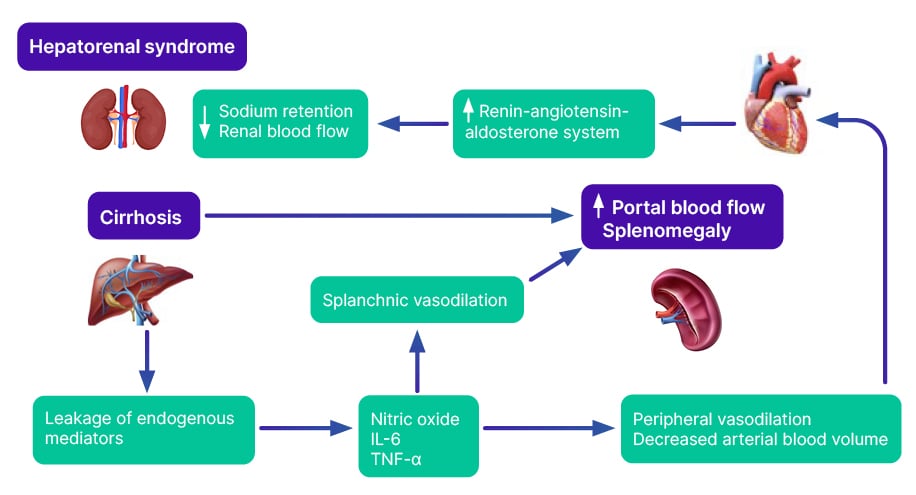
Figure 1: Entwined organ failure in liver cirrhosis.
Impact of end-stage liver disease on cardiovascular and pulmonary circulation
Splanchnic vasodilation due to liver cirrhosis leads to decreased systemic vascular resistance, causing relative hypotension.10 A possible mechanism is ‘leakage’ of endogenous mediators (nitric oxide, IL-6, and TNF-α) from the portal circulation into the systemic circulation, because of impaired hepatic metabolism. Further, increased catecholamines in cirrhosis can cause tachycardia and reduced cardiac afterload.11
Simultaneously, systemic vasodilation causes intrapulmonary vasodilation and portopulmonary hypertension.12 This can lead to ventilation-perfusion mismatch. The physiologically volume-compliant right ventricle dilates from the elevated right-sided pressure.13 Overall, cirrhosis can lead to right and left heart strain.
Impact on kidneys
Splanchnic vasodilatation leads to effective arterial intravascular volume depletion, activating the renin angiotensin aldosterone system and sympathetic pathways, resulting in renal vasoconstriction and decrease in glomerular filtration rate (GFR).14 As a compensatory mechanism, sodium reabsorption and passive fluid retention, reduced oncotic pressure, and non-osmotic release of vasopressin leads to increased extracellular water accumulation. Fluid retention and azotaemia can lead to cerebral oedema and intracranial hypertension.15 In many instances of cirrhosis, renal vasoconstriction and fluid shifts can lead to cumulative nephrotoxicity, and eventually interstitial fibrosis and needfor RRT.
IMPACT OF SURGERYON HOMEOSTASIS
Orthotopic LT surgery is a complex procedure that involves transplanting a donor liver to the recipient post-native hepatectomy. With associated AKI, there could be severe electrolyte and acid-base disturbances.
The surgery poses a substantial risk of developing de novo AKI from renal venous congestion (from clamping the inferior vena cava [IVC] and major veins) and ischaemic injury. Metabolic derangements can worsen intraoperatively if renal functionis compromised.
Liver Transplantation Surgeryand Hyponatraemia
Hyponatraemia is a common electrolyte abnormality seen in ESLD,16 and is associated with worse mortality in CKD.17 Sodium levels can rise due to improved liver function post-transplant. The anaesthesiologist can attempt to avoid substantial changes in sodium levels, but this might not always be possible because of the need for intravenous fluids and blood products. Rapid sodium correction can lead to osmotic demyelination syndrome.18
A study conducted by Romanovsky et al.,19 in LT, showed increased neurological deficits (p=0.006) if the 24-hour post-transplant correction was more than 10 mmol/L.
The goal before LT should be to improve the sodium level to avoid substantial changes intraoperatively. Hypotonic solutions for carrier fluids or volume resuscitation can be used to avoid overcorrection.18
Liver Transplantation Surgeryand Metabolic Acidosis
Lactic acidosis is common, and is associated with increased mortality in LT.20 The liver is an important organ in the ‘clearance’ of lactate. In states of hypoperfusion coupled with decreased clearance in liver dysfunction, this can be severe. Renal dysfunction further worsens metabolic acidosis due to losses of bicarbonate from the tubules and loss of renal buffer.
Under physiological conditions, the kidney is the second most efficient organ in removing/metabolising lactate.21 Studies have suggested that the kidney can be responsible for 20–30% removal of an exogenous load of lactate.22 A lot of the ‘removal’ of lactate by the kidney is by uptake and metabolism of lactate, rather than by urinary excretion.23 If there is metabolic acidosis, renal uptake of lactate increases despite a reduction in renal blood flow.24
Bicarbonate therapy in anuric AKI and lactic acidosis is unlikely to be beneficial, with the potential risk of volume overload. In addition, high volumes of parental bicarbonate can cause a paradoxical intracellular acidosis by increasing the concentration of dissolved CO2.25 Intracellular acidosis can decrease cardiac contractility.26
Intraoperative bicarbonate is not effective in lactic acidosis.27 RRT with bicarbonate-based dialysis baths can reduce acidosis, as perone study.28
Liver Transplantation Surgery and Portopulmonary Hypertension
Pulmonary artery hypertension associated with portal hypertension is called portopulmonary hypertension. All patients who are candidates for LT should be screened for portopulmonary hypertension by transthoracic echocardiography. Those with right ventricular systolic pressures greater than 45 mmHg should undergo right heart catheterisation.29 Mean pulmonary artery pressure greater than 35 mmHg is associated with increased operative risk, and should be treated prior to LT.30
Anaesthesia for LT has dramatically changed in the past three decades. As high-risk patients became candidates for LT, the risk for intraoperative complications has also increased. With the new MELD-NA scoring system, more patients with hyponatraemia are likely to be transplanted due to incorporation of the serum sodium in scoring. The risk of central pontine myelinolysis if serum sodium is corrected too rapidly is increased.31
All these variables, in conjunction with the use of more marginal organs, can result in more challenging management from an anaesthesia standpoint. Each stage during LT surgery incorporates its own set of challenges, and deep understanding of the physiologic derangements that each stage represents will decrease morbidity and mortality.
THREE STAGES OF SURGERY
Pre-anhepatic Stage
This stage starts from the surgical incision, and ends with freeing the native (diseased) liver from the vascular access by clamping the inferior vena cava (IVC), hepatic artery, and hepatic portal vein. Bleeding is common at this stage of surgery, and sometimes, massive transfusions are needed.
Surgical techniques currently used for LT include the conventional caval reconstruction technique. Conventional caval reconstruction involves recipient hepatectomy, including the retro-hepatic vena cava. This technique will cause occlusion of the vena cava and portal vein.32 This will result in a severe reduction in preload to the right heart during the anhepatic phase. Overcompensation with aggressive volume expansion can become detrimental, as this volume will return the circulation after unclamping. Hypervolaemia can lead to venous congestion and poor function in the newly transplanted graft. Second, the piggy-back technique involves hepatectomy with preservation of native retro-hepatic vena cava. This technique also has a few shortcomings, which include outflow obstruction, specifically in the hepatic venous cuff anastomosis.33 Haemodynamics are usually more stable than with a full clamp.
The application of veno-venous bypass is still justifiable in certain patient populations.34 With this technique, venous blood from the IVC and femoral vein is returned into the internal jugular vein using extracorporeal veno-venous cannulas and a centrifugal pump. This practice is decreasing due to lack of clinical evidence to support its use.35
Anhepatic Stage
The anhepatic stage begins with the occlusion of blood supply to the native liver (clamping the IVC), and ends with reperfusion of the new graft. Decreased blood flow below the diaphragm (from clamping the IVC) can cause worsening of metabolic acidosis.
In this phase, the new graft is anastomosed. Coagulopathy, hyperkalaemia, and acidemia are common, as the liver is rendered metabolically ineffective. The patient should be metabolically optimised by the anaesthesiologist to set up the stage for the reperfusion phase.36
Neohepatic Stage
This phase starts with reperfusion of the donor liver. As the IVC is ‘open’, venous blood will return to the right heart, with subsequent improvement in cardiac output. However, this improvement in haemodynamics will come at a cost. After the portal vein is open, acidotic and hyperkalaemic blood from below the clamp and from the graft itself will return to the right heart.31 In addition, toxins that have been accumulating in the enteric system while the portal vein is clamped, will be reintroduced into the general circulation.37 Severe hypotension upon unclamping is called reperfusion syndrome and can happen in up to 12.1–31.6% of patients.38-40 This can improve with the administration of calcium chloride, bicarbonate, and vasopressors.31
ROLE OF CONTINOUS RENAL REPLACEMENT THERAPY DURING LIVER TRANSPLANT
Risk factors for AKI during surgery include a full caval clamp, long anhepatic time, or prolonged hypotension. A selected group of patients with volume overload, hyperkalaemia, hyponatraemia, or preoperative AKI may benefit from the use of CRRT that can be started in the operating room or after arrival to the intensive care unit.31 RRT in AKI can not only control electrolytes and acid-base status, but can also avoid positive fluid balance, which is associated with adverse outcomes in critically ill patients.
CRRT provides enhanced haemodynamic tolerance, sustained acid-base, and azotaemia control. The premise on the use of intraoperative CRRT in LT is based on the rationale that patients with impaired kidney function will poorly tolerate fluid shifts and metabolic disturbances during the intraoperative period. The use of intraoperative CRRT has been proven safe with no significant difference in complications, compared with patients that underwent a classicapproach (Table 1).41
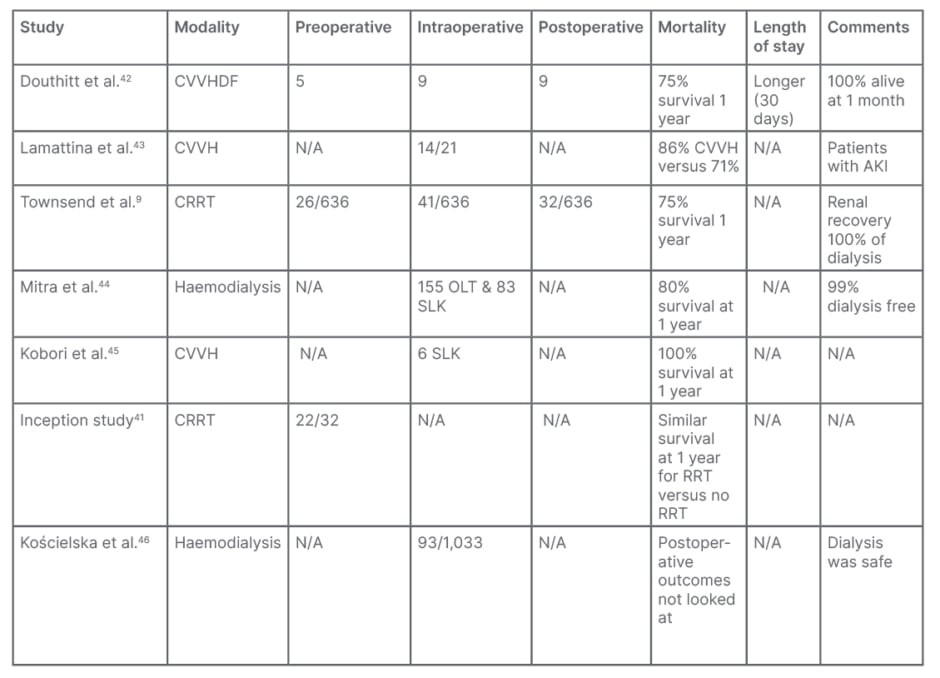
Table 1: Summary of studies on perioperative renal replacement therapy in liver transplantation.
AKI: acute kidney injury; CRRT: continuous renal replacement therapy; CVVH: continuous veno-venous haemofiltration; CVVHDF: continuous veno-venous haemodiafiltration; N/A: not applicable; OLT: orthotopic liver transplant; SLK: simultaneous liver kidney.
From these studies, the authors can conclude that perioperative (including intraoperative) RRT is feasible and safe, with outcomesthat were not significantly worse than in those who did not need it. The modality most used was CRRT, except one study, which used intermittent dialysis.
There are risks and benefits of each modality of RRT. Table 2 briefly summarises the advantages and disadvantages of intermittent and continuous modalities of dialysis.
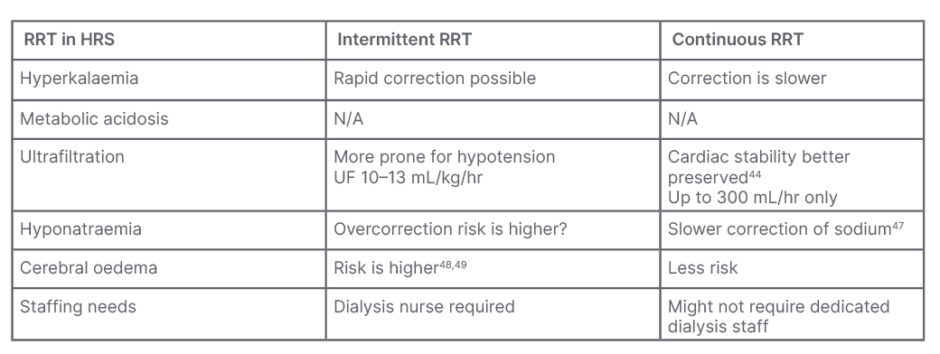
Table 2: Advantages and disadvantages of intermittent and continous modalities during surgery.
HRS: hepatorenal syndrome; N/A: not applicable; RRT: renal replacement therapy.
Liver Transplant
In 2023, 10,660 LTs were done in the USA, as per UNOS data.50
Benefits of Preoperative CRRTin Liver Transplant
RRT can correct electrolyte and acid-base abnormalities, and optimise volume status by ultrafiltration in the setting of fluid overload.
Benefits and Challenges ofContinuous Renal ReplacementTherapy Intraoperatively
As previously described, it is possible intraoperatively to develop acidemia, hyperkalaemia, rapid correction of hyponatraemia. RRT can help prevent this or at least limit the severity of these electrolyte and acid-base issues.
Bleeding is common during surgery, and the need for blood transfusions (sometimes massive transfusions) is common.51 This can lead to significantly lowered filling pressures. Volume expansion should be a balance between maintaining a low central venous pressure and maintaining adequate cardiac filling pressures in the setting of ongoing blood loss.
Clotting of dialysis filters is an amplified complication of CRRT here, because of decreased anticoagulation use, due to high bleeding risk with heparin. Citrate-based anticoagulation should be used with caution in liver disease, due to unpredictable metabolic clearance.52 Citrate accumulation can also occur when the rate of citrate metabolism exceeds hepatic metabolism, leading to metabolic alkalosis.53
In one study,54 citrate-based anticoagulation was done in patients with liver disease with close monitoring of acid-base parameters. They identified that a prothrombin time ≤26% and a serum lactate level ≥3.4 mmol/L was useful for predicting citrate accumulation. A reduced dose of citrate infusion might be warranted inliver disease.
Methods to offset circuit clotting
Methods to offset circuit clotting include: flushing the extracorporeal circuit with normal saline every 30–60 minutes; use of rostacyclin as anticoagulation in the extracorporeal circuit;55 and use of serine protease inhibitors nafamostat mesylate and kallikrein.56
Intermittent Haemodialysis DuringLiver Transplant Surgery
Most of the studies in Table 1 used CRRT during surgery. Life-threatening hyperkalaemia or acidosis that develops intraoperatively can be corrected by haemodialysis more rapidly.
The study by Mitra et al.45 noted 1-year survival of 80% in patients who had intraoperative haemodialysis. The concern with intermittent dialysis is hypotension and neurological complications from rapid solute shiftsand hypotension.
This study showed that intraoperative haemodialysis is possible, though it must be noted that unstable patients were on CRRT before surgery in that study. In patients with an arteriovenous fistula, sustained low efficiency dialysis can be a safe option to avoid dialysis catheter-related infections. Sustained low efficiency dialysis can also be a safer alternative to intermittent haemodialysis intraoperatively.
Assessing Renal Recovery Post-op AKI is a common complication post-LT.57 Some of the risk factors associated with post-LT AKI are unique from other medical and surgical causes of AKI.58 These include hepatic reperfusion injury and marginal graft use.59 Assessment of renal recovery could be hampered if liver function is impaired, as creatinine-based estimated GFR can be overestimated in liver dysfunction.60 AKI based on urine output or cystatin-based measurements can be more accurate to assess renal solute clearance.61
One study showed renal recovery of AKI post-LT was 33% among those who had an estimated GFR <30 mL/min (MDRD6).62
CONCLUSION
ESLD is associated with high mortality. LT is the definitive treatment and is a complex surgery. The risk of renal dysfunction with liver cirrhosis during the surgery is high. Perioperative RRT could help correct or prevent some of the electrolyte and acid-base abnormalities encounteredduring surgery.
There are challenges with RRT during LT, but the authors reviewed studies that have shown that it is safe, feasible, and with potential benefits. This would require interdisciplinary co-ordination and expertise, but the benefits include less complications, better outcomes, and possibly even decreased length of stay. Defined protocols for implementing intraoperative RRT should include vascular access, modality of RRT, timing of initiation (how soon during surgery),and anticoagulation.







