Summary
Severe asthma (SA), chronic rhinosinusitis with nasal polyps (CRSwNP), and eosinophilic granulomatosis with polyangiitis (EGPA) are three conditions driven by IL-5 and eosinophilic inflammation. As these conditions have high use of healthcare services, as well as lifestyle and psychological burdens, well-tolerated treatment to achieve optimal control is key. In all three conditions, as for many other eosinophilic diseases (ED), oral corticosteroids (OCS) are often used for both acute and maintenance treatment where disease activity is high. While, in general, OCS are very effective, their use is limited by a well-recognised high potential for adverse effects (AE). Moreover, cumulative exposure to OCS may not be acknowledged in many patients, especially for those predominantly treated in primary care, exposing patients to potentially damaging long-term OCS-related AEs. To discuss the use of OCS for these eosinophilic diseases, as well as to provide guidance on how to help limit their use, a board of European experts within each field was gathered. The experts completed questionnaires regarding treatment and referral pathways for patients with SA, CRSwNP, or EGPA; then, in an online meeting, discussed a number of issues in regard to OCS use. Here, the authors present the key recommendations from the expert advisory panel alongside some background to these conditions regarding treatment with OCS.
INTRODUCTION
Globally, systemic corticosteroid use for EDs is widespread.1 An expert panel of advisors was convened to discuss OCS use, overuse, and ways to limit use for patients with EDs such as SA, CRSwNP, and EGPA, all populations where cumulative OCS doses may be high. Discussion included how such use depends on several factors, including disease severity and phase, organ involvement, comorbidities, responsivity to therapy, and treatment adherence. These discussions have implications for other EDs such as hypereosinophilic syndrome. This typically multi-organ and tissue damaging disease is also predominantly treated with OCS, kinase inhibitors, and biologics; and similar issues with long-term use of OCS, as discussed below, may apply to this and all EDs where such treatment is common.2 Unless specifically referenced, the opinions shared here are those of the advisors.
ORAL CORTICOSTEROID DOSE AND ADVERSE EVENT PROFILE
As with most illnesses, treatment decisions regarding OCS use should be individualised to the patient’s needs and account for disease, behavioural, comorbidities, and environmental factors. There is no standard definition of what constitutes a low or high dose of OCS, or an acceptable lifetime cumulative dose, the advisors discussed, so there may be misconceptions in clinical practice with detrimental consequences regarding morbidity and mortality, even for patients receiving a low OCS dose. Recently, a European League Against Rheumatism (EULAR) task force deemed that for maintenance OCS use, there was a low risk of harm at a dose of ≤5 mg/day, a need for individual harm assessment at >5−10 mg/day, and an elevated risk of harm at >10 mg/day.3 However, the advisors stressed how cumulative exposure, including for other inflammatory conditions, must be taken into account, as in one long-term study including 24,117 adults with SA, adverse outcomes began with cumulative OCS exposures of 500−1,000 mg, equivalent to four OCS courses over a lifetime,4 and, pointed one of the advisors, lower than the annual dose for a patient on 5 mg/day.
While efficacious, there are myriad AEs associated with OCS exposure (Figure 1), as they regulate inflammation and immune function; lipid, carbohydrate and protein metabolism; brain function; calcium and bone metabolism; and cardiovascular homeostasis.5,15,16 For example, OCS action on the anterior pituitary gland and hypothalamus leads to decreased release of associated hormones and, subsequently, potentially iatrogenic adrenal insufficiency.6,17 Another example is psychological effects, with one study finding a high incidence rate difference for depression and anxiety between OCS and non-OCS users with asthma, even in those with low OCS cumulative exposure. Of note though, this may not be a direct causative effect.18
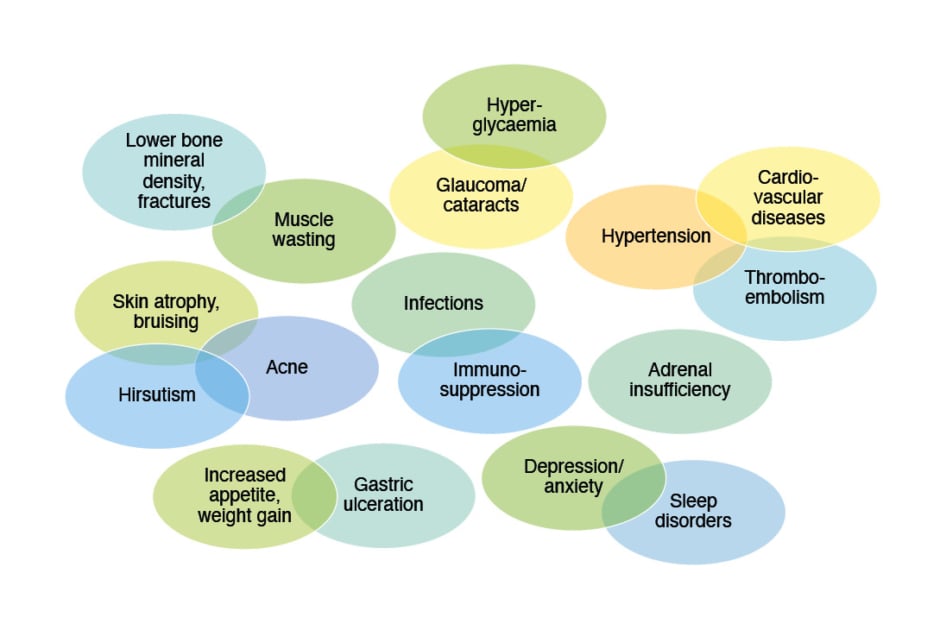
Figure 1: Adverse events associated with oral corticosteroid use.5-15
Also of concern to the advisors is mortality associated with OCS use. Most studies here have been carried out in patients with asthma. For example, in a South Korean study including 16,668 OCS-dependent and matched OCS-independent adults with asthma, there was a hazard ratio (HR) of mortality of 2.17 (95% confidence interval: 2.04−2.31) with HR increasing with dose.19 A Swedish study including 217,993 patients with asthma aged ≥6 years found an HR for mortality of 1.34 (95% confidence interval: 1.24−1.45) for regular OCS users compared to non- or periodic-OCS users.20
Patients themselves have concerns regarding OCS use due to AEs, with one study of adult patients with asthma reporting that 44% of 268 patients had a ‘negative image’ of OCS, with 26% reducing or stopping OCS treatment, and 42% and 34%, respectively of 230 patients, saying they would try to find an alternative treatment and would ask advice from another healthcare professional (HCP).21
TREATMENT OF EOSINOPHILIC DISEASES WITH ORAL CORTICOSTEROIDS
Severe Asthma
Approximately 5−10% of the 300 million people worldwide with asthma are diagnosed with SA, which may involve frequent exacerbations and hospitalisations, or have at least one severe exacerbation per year.22-25
Morbidity and mortality rates are high in SA and burdens include healthcare costs, corticosteroid-induced comorbidity, psychological factors, and work-related difficulties.23,26-29 SA assessment and management should preferably be carried out in a specialised centre, in an individualised step-wise approach, with validation of diagnosis and attention to comorbidities. The Global Initiative for Asthma (GINA) has SA treatment goals, including good symptom control and risk reduction with minimal treatment side-effects. For patients aged ≥12 years old they recommend medium/high dose maintenance inhaled corticosteroids (ICS) plus formoterol or, as an alternative reliever, long-acting β-2 agonists, and considerations for adding a long-acting muscarinic antagonist and a biologic treatment if needed. They recommend only short courses of OCS for severely uncontrolled asthma, with maintenance OCS ‘as last resort’, and place high priority on OCS minimisation strategies.30
Studies investigating OCS use for patients (>5 years) with SA show that OCS mean daily maintenance doses range from 4.0−21.4 mg prednisolone equivalent,1 with daily doses of 5.5−7.5 mg for patients prescribed OCS for ≥2 years.31 Major reasons healthcare professionals (HCP) state for maintenance OCS prescription include relative resistance to ICS and other controller medications, increased numbers of exacerbations, and increased disease severity.1 The advisors highlighted the need to perform routine inhaler adherence and use checks, and discussed how worsening symptoms may be automatically attributed to poor asthma control when there are other potential causes. In these latter situations, patients have a high risk of being prescribed a rescue course of OCS without objective signs of worsening such as a decline in lung function.
Despite biologic treatment availability for SA, the advisors discussed how OCS use continues to be high.1,32 For instance, a survey including 4,990 adult patients from the International Severe Asthma Registry (ISAR) found that 63.1% of patients in Italy, 59.6% in the UK, 23.3% in the USA, 24.7% in Australasia, and 20.7% in South Korea received maintenance OCS treatment at registry; although, the advisors noted, many of these patients will have subsequently proceeded to biologic therapy in specialist centres.24 A study including 1.7 million Spanish patients (≥12 years) showed that of the 5.5% of the study population who had asthma, 7.7% of these had SA, with nearly a third of these patients being OCS-dependent.25 In a Portuguese study, 91.3% of 46 physicians with a SA speciality thought that OCS use was necessary to control SA, and approaching two-thirds did not consider that there was maintenance OCS overuse.33
Both long- and short-term OCS use is associated with increased healthcare costs for people with asthma compared to no use, as is long-term use compared to short-term.1 For instance, in one study, patients on maintenance OCS had 43% more costs than those not receiving this, partially due to cost of medications to manage OCS-related AEs.34 Indeed, another study showed such costs to be higher in patients with SA with high OCS exposure compared to patients with mild/moderate asthma with low OCS exposure.35
Chronic Rhinosinusitis with Nasal Polyps
In CRSwNP, which has an estimated global prevalence of 1−4%, nasal inflammation can be accompanied by loss/reduction of smell; nasal blockage, discharge, and congestion; facial pain/pressure; and polyps that can lead to partial or complete nasal obstruction.36-39 CRSwNP, compared to people without this disease and people with chronic rhinosinusitis without nasal polyps, is associated with greater symptom burden and medication use, along with missed workdays and decreased productivity and quality of life (QoL).36,38,39 CRSwNP and asthma are often comorbid, occurring in around 40% of people with either condition.40,41 Such comorbidity is associated with higher OCS use, including number of courses per year and rate of maintenance therapy.40
Surgery may be needed for severe CRSwNP; however, post-operative polyp recurrence is high, particularly in those with eosinophilic polyps and patients with comorbid asthma.36,42 More recently, biologic therapy with anti-IL4Rα has been recommended in some patients, including those who need ≥2 courses of OCS/year or OCS therapy >3 months.36 While localised, intranasal, steroid treatment is routinely prescribed for CRSwNP, short courses of OCS may reduce polyp size.36,43-45 The advisors noted though that in recent years, this has not been recommended, even if limited to 1−2 courses/year. Studies of 7−21 days of OCS for CRSwNP, usually additional to intranasal corticosteroids, show that therapy led to reduced symptoms, including improved sense of smell and nasal flow, and decreased nasal polyp scores. The latter can remain decreased for several weeks after therapy initiation; however, symptoms typically relapse.36,44,46,47 Evidence is conflicting as to whether OCS compared to nasal corticosteroids following CRSwNP surgery is beneficial, with some studies showing greater effects and lower recurrence rates, and others not showing an advantage.48-50
According to the advisors, OCS use for CRSwNP is generally symptom-driven, not preventative. Such therapy is infrequent in some countries, with OCS being prescribed only prior to surgery or as rescue medication for severe uncontrolled symptoms.51 However, an Italian study including 437 otorhinolaryngologists found that 94% prescribed a short course of OCS for CRSwNP re-exacerbation, with only 41.1% saying they do not exceed two courses/year and 13.4% not exceeding four courses/year. While 35.0% considered 4 weeks/year to be the cut-off point for high risk for AEs, 16.9% considered the cut-off to be 8 weeks/year. Total yearly dose considered dangerous was 1,000 mg for 23.1% of respondents and 2,000 mg for 11.2% of respondents.52
Eosinophilic Granulomatosis with Polyangiitis
The immune-mediated inflammatory disease EGPA, a form of small-vessel, necrotising, anti-neutrophil cytoplasm antibodies-associated vasculitis, has an estimated annual incidence of 0.5−6.8 cases/million.53-56 While disease course may differ between patients, EGPA typically progresses slowly from early phase asthma, sinusitis, and rhinitis, followed by tissue infiltration by eosinophils and vasculitis. There can be pulmonary, renal, and gastrointestinal involvement; peripheral nervous system damage; and eosinophilia-driven cardiomyopathy.53,57
The advisors saw the primary goal of EGPA therapy to be to induce remission and prevent tissue/organ damage. Subsequent management goals include relapse prevention, minimisation of OCS and immunosuppressant use, and QoL improvement. In EGPA guidelines, OCS therapy recommendations include starting with 2−3 weeks’ 1 mg/kg/day prednisolone, tapered to 0.3, then 0.15 mg/kg/day after 3 and 6 months, respectively, to achieve a minimally effective dose or withdrawal.55 While maintenance doses are ideally <7.5 mg/day prednisolone,55 one study found that a maintenance daily dose of 12.9±12.5 mg was needed to control EGPA symptoms.58 Such OCS therapy is associated with improved remission and survival rates.56,59-62 Intravenous steroids may be administered in life or organ-threatening cases.56,62 For those with more severe EGPA, therapy may include cytotoxic agents such as cyclophosphamide, anti-IL-5 agents, B cell depletion therapies, or other biologics.55,56,62,63 Asthma symptoms in EGPA, a major cause of chronic OCS use in these patients, are treated as for this condition, according to GINA recommendations.30,56,62
The advisors highlighted that in general, high doses of OCS for maintenance treatment potentially result from a lack of early referral to the appropriate specialist. As such, there is a need for education among HCPs regarding EGPA screening, early diagnosis, and treatment and risks of prolonged OCS use. OCS can be used for emergency EGPA treatment, but there should be no minimal level considered non-harmful as this may ‘encourage’ OCS prescription, albeit at a low level. However, one advisor noted that it is difficult to avoid OCS use for some patients due to recurrence of symptoms and exacerbations. Another advisor stressed that the need for an OCS course should trigger HCPs to review other potential causes of disease recurrence (e.g., incorrect inhaler technique, infection, or poor treatment adherence).
INITIATIVES TO REDUCE BURDEN OF ORAL CORTICOSTEROIDS
Overall, the advisors agreed that OCS use for these EDs should be reserved for intermittent/rescue therapy only, at the lowest dose possible if there are disease flares (e.g., 1−2 courses a year at 0.5−1.0 mg/kg prednisolone or equivalent for 2 weeks maximum). OCS should be eliminated in the maintenance setting or, if unavoidable due to the need for disease control, prescribed at the lowest possible dose. Measures of success regarding OCS reduction included either no maintenance OCS or reduced OCS use (by 50−70%; 1−2 courses/year), and reductions in symptom and adverse QoL measures.
As can be seen from Figure 2, the advisors suggested several initiatives to reduce OCS burden.
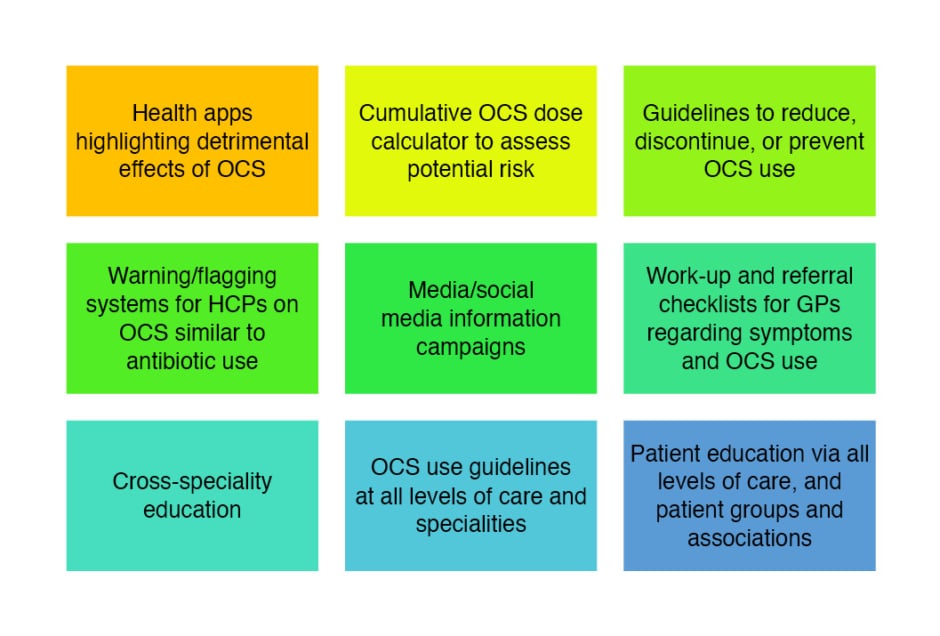
Figure 2: Potential initiatives to reduce oral corticosteroids burden.
GP: general practitioner; HCPs: healthcare professionals; OCS: oral corticosteroids.
Cumulative Dose Risk
While there is a lack of evidence as to what an acceptable cumulative OCS dose is, most advisors agreed that the maximum lifetime dose should be 500−1,000 mg and that the ideal is no more than three courses over a lifetime (depending on length of course) to mitigate severe AEs. For patients who do require long-term treatment, concerns especially included administering a dose >5.0−7.5 mg/day, although even lower doses were of concern; or using OCS for patients with comorbidities that may be aggravated by such OCS use.
The more a patient is exposed to OCS over their lifetime, the advisors discussed, the more complications they may experience later in life. This is important as it was noted that patients may not be asked about, or properly recall, previous OCS use. Therefore, calculating cumulative lifetime exposure may be problematic. While a patient’s maintenance OCS prescription history may be checked with electronic prescribing records, as-needed OCS use may be harder to track as the patient may not report it, and one advisor noted that OCS are available over the counter and/or patients self-administer in some countries. Tracking of cumulative OCS exposure also becomes harder where there is countrywide heterogeneity of electronic patient records and patients move hospital and/or primary care provider (PCP). Additionally, the advisors discussed, not all countries have electronic recording, so patient reporting may be the main way to evaluate OCS use. This can also be difficult as patients may not always know medication details. For example, one advisor discussed a patient who was administered what was described by their PCP as an ‘allergy vaccination’, which was actually an OCS.
Healthcare Professional Education and Policy Maker Involvement
A large need was seen by the advisors to educate all HCPs, especially in primary care, regarding treatment options other than OCS for these EDs. However, one noted, OCS are cheap and easy to prescribe, so other options such as biologics could be seen to impact a PCP’s budget. Additionally, some countries prohibit PCPs from receiving direct information from manufacturers regarding biologics. Another problem the advisors highlighted was how non-specialists may prescribe OCS more broadly due to lack of awareness regarding cumulative negative impact on health outcomes. OCS may also be prescribed for an acute exacerbation of an ED, but the patient is not always followed-up to assess frequency of such OCS prescription or potential AEs. This may be due to restricted capacity within primary care. In these cases, the advisors discussed how specialists need to work with and inform a patient’s PCP to help reduce the dose or withdraw OCS use, and, ultimately, the advisors recommended that SA, CRSwNP, and EGPA are treated in specialist centres to avoid indiscriminate OCS administration.
Further discussion centred on the need for multidisciplinary team patient care networks, with PCP and specialist involvement, and broad HCP meetings and educational initiatives regarding OCS use. This could include multidisciplinary team workshops and round table discussions, structured patient interviews by expert clinicians at symposia, and collaborative programs with PCPs seeing patients together with a specialist.
One strategy suggested by the advisors to improve awareness regarding reducing OCS use in these EDs included educating policymakers regarding unmet needs, to facilitate changes on a national level. Organisers of healthcare systems and insurance companies, where applicable, also need to be aware of the need to use drugs with more favourable safety profiles than OCS, and data is needed to show the impact of using therapies other than OCS.
Referral Checklists
One issue with OCS use, according to the advisors, may be because secondary referral pathways for eosinophilic diseases are not optimal. For instance, a UK study revealed that 72% of 16,409 patients (≥16 years) identified with potential SA in primary care had not been referred to a specialist in the past year.64 Referral checklists could be beneficial to ensure that patients requiring specialist care are referred appropriately and early during the disease course. One advisor suggested developing protocols to ensure patients are referred to specialists before being prescribed maintenance OCS.
Monitoring parameters or triggers for specialist referrals could include not only disease-related factors, but also, according to the advisors, OCS-related prompts, including repeat prescription of OCS and/or injectable steroids, potential steroid-associated AEs, and high-dose OCS use. A warning system could be linked to a patient’s electronic medical records to notify PCPs when patients receive, for example, more than two courses of OCS/year, as is done in the UK to flag excessive short-acting β2-agonists use. However, it was discussed how the feasibility of such initiative is not clear due to variability between healthcare systems in their ability to have a red-flag system on a patient level basis and, in some cases, heterogeneity in software packages used across a country.
Patient Education
Including patients in OCS reduction initiatives is key, as withdrawal effects such as adrenal suppression can keep patients dependent on OCS.33 Suggestions from the advisors included patient education via videos in a clinic/PCP waiting room, and patient congresses and online meetings. Patients should also be advised on ways to minimise AE risks (e.g., avoiding weight gain, exercising to aid bone protection, and cardiovascular AEs).
The advisors suggested that patients could complete an AE symptom checklist under supervision of their PCP, as many patients recognise OCS-related AEs but may not be aware of alternative treatments. The Glucocorticoid Toxicity Index (GTI), for example, can track changes in several domains, including glucose tolerance, bone mass index, skin toxicity, bone density, infection, and neuropsychiatric symptoms.65 Patients reporting symptoms and OCS use via health apps is feasible; however, one advisor reported how such apps had not engaged their patients, with many only using them for a couple of days. Such health apps, it was discussed, are also burdened with complexity around data protection and relevant analysis being carried out by commercial companies.
Oral Corticosteroids Limitation More Specific for severe Asthma, Chronic Rhinosinusitis with Nasal Polyps, or Eosinophilic Granulomatosis with Polyangiitis
In specific regard to SA, the advisors noted how, with the advent of biologics, it is possible to maintain disease control without the use of OCS or using only the smallest dose that maintains disease remission in combination with QoL. They proposed a systematic approach to OCS dose limitation that assesses and improves medication adherence, provides education on ICS device technique, provides an asthma self-management plan, optimises asthma treatment, and manages comorbidities. Such a strategy is associated with exacerbation reductions, greater symptom control, increase in lung function, improved QoL, and significant reduction in OCS dose.66 Once OCS use was reduced/eliminated, the advisors discussed how ICS use should also be tailored to individual needs.
While OCS are recommended in CRSwNP guidelines,36,45 the advisors noted that there is little information regarding dose regimens and duration of treatment, and there are few studies regarding minimal dose for maintenance treatment. Although some advisors reported they would consider alternative treatment options if patients with CRSwNP have >2 courses of OCS/year, others indicated that one course is sufficient to warrant treatment change or escalation to biologics, and it was underlined how HCPs need to be more aggressive about limiting OCS use. However, one advisor warned against complete ‘steroid phobia’ as OCS are relatively inexpensive and effective in cases with respiratory airway involvement. Another suggestion was that PCPs managing CRSwNP should attend specialist-led training events to educate them regarding OCS use.
In cases of EGPA with clear vasculitis manifestations, the advisors discussed how OCS use, plus biologics such as anti-IL-5 or immunosuppressive agents, may be necessary for maintenance therapy. A dose of ≤5 mg/day prednisolone was deemed potentially acceptable (although, it was noted, this is still a large cumulative dose) or the minimal dose needed so a patient has <3 exacerbations/year, and a view to tapering off when possible. Increased awareness is needed regarding comorbidities that could represent disease treatable traits and, if there is other vital organ involvement, careful OCS dose tapering to a minimal dose is required. Age is also a factor of concern for the advisors as younger patients may have a higher risk of being on maintenance treatment for longer time periods. One advisor highlighted how, “in anything but EGPA, rheumatologists do not accept long term OCS treatment.” As such, alternatives to OCS use should be examined for all patients and there is a need for early, steroid sparing pathways. Of note though, complete OCS removal for some patients is not feasible as this may lead to exacerbations.
CONCLUSION
Although there are alternatives to OCS treatment, the advisors discussed that for SA, CRSwNP, and EGPA, many patients are prescribed intermittent and/or maintenance OCS therapy despite the known AE profile. They stressed how education and initiatives are needed to increase awareness regarding OCS AEs and underline the necessity to focus on optimal efficacy with minimal toxicity. These will help reduce OCS use to only when necessary and, if needed, then at the lowest dose possible to maintain an effect and limit AEs.








