Abstract
Background: Multifocal motor neuropathy (MMN) is a rare immune-mediated disorder characterised by progressive, asymmetric muscle weakness primarily affecting distal limb nerves without sensory involvement. While MMN is estimated to affect only one-to-two individuals per 100,000, the presence of anti-GM2 antibodies in MMN is exceedingly rare, with fewer than five reported cases in the medical literature.
Case presentation: This case report describes a 51-year-old female with a history of ankylosing spondylarthritis and Crohn’s disease who developed left wrist weakness and sensory disturbances following infliximab infusion and a SARS-CoV-2 vaccination. Electromyography revealed neurogenic changes and motor conduction block in the left radial nerve, which is consistent with the MMN diagnosis. Further investigation showed elevated anti-GM2 antibodies in serum, a rare finding associated with MMN. Treatment with intravenous immunoglobulin (IVIg) resulted in significant symptom improvement.
Conclusion: This case highlights the diagnostic challenges in MMN associated with anti-GM2 antibodies, emphasising the importance of early recognition and targeted management in similar clinical presentations. Although the patient’s symptoms improved after IVIg treatment, the aetiology of MMN and the role of anti-GM2 antibodies remained unclear.
Key Points
1. Multifocal motor neuropathy (MMN) is a rare immune-mediated disorder affecting one-to-two per 100,000 individuals, with anti-GM2 antibodies being exceedingly rare, making early recognition and management critical for preventing long-term disability.
2. The authors present the case of a 51-year-old female with MMN associated with anti-GM2 antibodies following infliximab infusion and COVID-19 vaccination, highlighting diagnostic challenges and treatment response to intravenous immunoglobulin.
3. Clinicians should consider MMN in patients with progressive asymmetric weakness, even with atypical antibody profiles, and explore potential links between vaccines, immunomodulatory therapies, and autoimmune neuropathies.
INTRODUCTION
Multifocal motor neuropathy (MMN) is a rare immune-mediated condition recently documented in the medical literature. MMN typically manifests with gradual, progressive, asymmetric muscle weakness in the distal limbs, predominantly affecting the ulnar, median, radial, and tibial nerves.1-4 MMN has been found to affect predominantly men, with a male-to-female ratio of approximately 3:1, and typically manifests around the age of 40. Prevalence rates vary globally, with estimates ranging from 0.6 to 2 cases per 100,000 individuals, with no significant differences reported across ethnicities.1-3 A defining electrophysiological feature of MMN is multifocal chronic conduction block (CB) affecting motor neurons only.5-7 MMN is thought to result from an autoimmune attack on peripheral motor nerves, mediated by antibodies targeting gangliosides such as GM1, GD1a, and, rarely, GM2. Approximately 30–80% of patients with MMN exhibit elevated levels of IgM antibodies against GM1, and less commonly GM2 and GD1a, detectable in their blood.8 These antibodies are thought to disrupt nerve conduction by targeting gangliosides in the myelin sheath of motor nerves, and their disruption leads to conduction block and muscle weakness. Intravenous immunoglobulin (IVIg) therapy is highly effective in treating MMN. IVIg works by modulating the immune system, neutralising autoantibodies, and suppressing inflammatory pathways. Multiple studies have demonstrated its efficacy, with significant improvement in muscle strength observed in up to 70–80% of patients.9,10 However, up to 20% of patients with MMN experience moderate-to-severe impairment, primarily in the upper limbs.1
PATIENT INFORMATION
A 51-year-old female with a medical history of ankylosing spondylarthritis and Crohn’s disease presented with left wrist weakness and sensory disturbances following infliximab (originator) infusion and SARS-CoV-2 vaccination. The patient had been previously treated with three doses of infliximab biosimilar, at the standard dose of 5 mg/kg every 8 weeks without adverse effects. Due to a drug shortage in Lebanon, she was switched to infliximab originator at the same dose. However, during the first 30 minutes of infliximab infusion, she developed generalised pruritus and a rash on her hands, chest, and back, a reaction that occurs in approximately 3–10% of patients receiving infliximab.11
Treatment with cortisone and desloratadine resulted in the disappearance of itching and rash after approximately one hour, except for persistent itching on the soles of her feet; therefore, the infusion was allowed to continue after this time. Three weeks after the infusion, she presented with left wrist weakness, tingling sensations in her feet, hypoesthesia resembling walking on cotton, and calf contractures. Notably, 20 days prior to infliximab infusion, the patient had completed a course of treatment with cefixime 400 mg (0-1-0) and metronidazole 500 mg (1-0-1) for severe gastroenteritis exacerbated by consumption of raw meat. Additionally, the patient had received two doses of the Pfizer-BioNTech COVID-19 vaccine, with the second dose administered 3 days prior to her last infliximab infusion.
CLINICAL FINDINGS
On physical examination, motor strength was intact in bilateral upper and lower limbs except for left wrist extension weakness (3+/5). Reflex testing revealed diminished responses in the left triceps (0/4) compared to the right triceps, biceps, brachioradialis, patellar, and Achilles tendon (2/4). Sensory examination indicated preserved vibration sense over toes bilaterally and intact light touch at the knees and fingertips bilaterally. Upper extremity sensory testing showed diminished light touch sensation in the left hand, particularly over the dorsum.
TIMELINE
Day 0: Received second dose of Pfizer-BioNTech COVID-19 vaccine.
Day 3: Infusion of infliximab originator after a drug shortage of infliximab biosimilar.
Day 23: Onset of left wrist weakness and sensory disturbances.
Day 25: Initial electromyography (EMG) showing conduction block in the left radial nerve (Figure 1).
Day 32: Second EMG confirming motor conduction block in both radial nerves (Figure 2).
Day 60: Third EMG showing resolution of conduction blocks but persistent fibrillations (Figure 3).
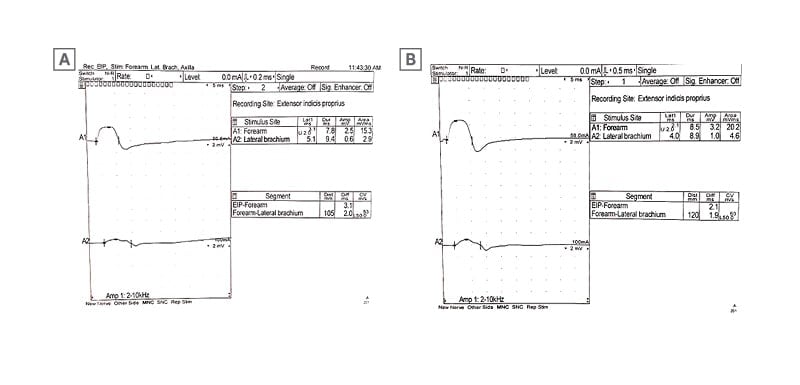
Figure 1: The initial electromyography findings obtained 2 weeks after symptom onset.
A) Motor nerve conduction study of the left radial nerve shows there is a conduction block at the lateral arm stimulation point. B) Motor nerve conduction study of the right radial nerve shows there is a conduction block at the lateral arm stimulation point.
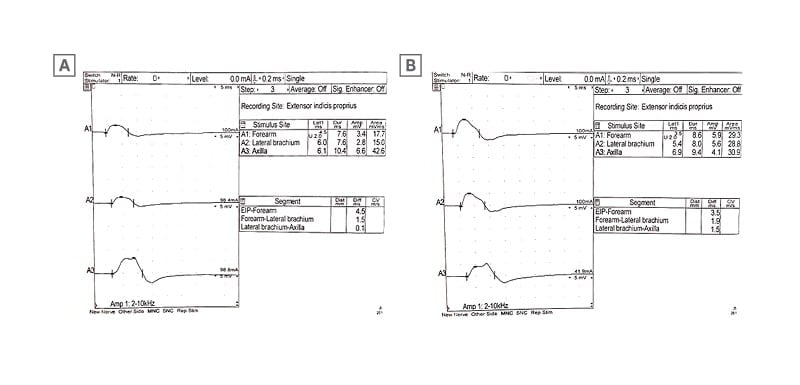
Figure 2: The electromyography findings obtained 4 weeks after IVIg treatment.
A) Motor nerve conduction study of the left radial nerve shows there is a disappearance of the conduction block at the lateral arm stimulation point. B) Motor nerve conduction study of the right radial nerve shows there is a disappearance of the conduction block at the lateral arm stimulation point.
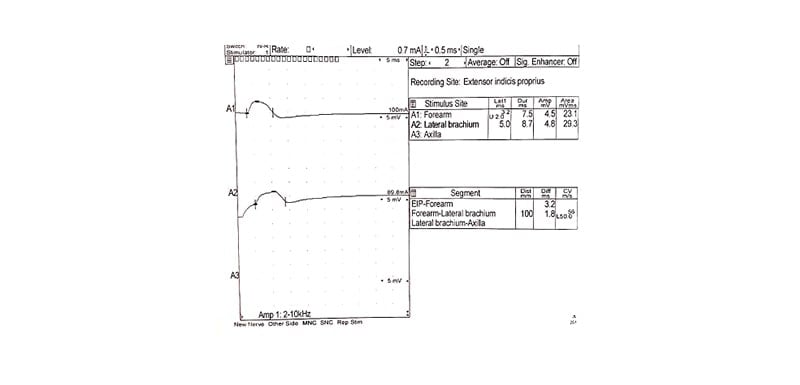
Figure 3: Illustrating the third electromyography findings 8 weeks post-treatment.
Motor nerve conduction study of the left radial shows no conduction block.
DIAGNOSTIC ASSESSMENT
The patient was evaluated with EMG, which showed a normal sensory study but reduced recruitment patterns and neurogenic motor unit changes in the left extensor indicis propius, with a suspected conduction block in the left radial nerve (Figure 1). Two days later, the patient presented to the ER for further degradation of her left wrist extension. Motor exam showed left wrist extension of 3-/5 with an absent left triceps reflex, which was also present in the initial physical exam.
Lumbar puncture was performed, and cerebrospinal fluid (CSF) analysis showed that the protein level was 0.35 g/L (normal values: 0.12–0.6 g/L), while the white blood cell count was 9×106 /L (normal values: 0–5×106 /L). Lab workup, including CBC, biochemistry panel, C3, C4, anti-Helicobacter pylori antibody, parathyroid hormone, brucellosis antibodies, rheumatoid factor, folic acid, vitamin B12, vitamin D, TSH, Widal test, antinuclear antibody (ANA), anti-Sjögren’s syndrome-related antigen A (anti-SSA; also called anti-Ro), anti-Sjögren’s syndrome-related antigen B (anti-SSB; also called anti-La), anti-Smith antibody (anti-Sm), anti-ribonucleoprotein/Smith antibody (anti-RNP/Sm), anti-scleroderma 70 antibodies or anti-topoisomerase I antibody (anti-Scl 70; associated with systemic sclerosis), anti-histidyl-tRNA synthetase antibody (anti-Jo 1; associated with polymyositis and dermatomyositis), anti-double-stranded DNA immunoglobulin G (anti-dsDNA IgG; highly specific for systemic lupus erythematosus), and anti Campylobacter jejuni and fetus, were within normal range.
Anti-ganglioside antibody analysis of the serum revealed high levels of anti-GM2 with 102 (reference range: 0–30). The blots for other anti-gangliosides (anti-GM1, anti-GD1a, anti-GQ1b, anti-GD1b, and anti-MAG) were negative.
THERAPEUTIC INTERVENTION
The patient was treated 1 month after the initial symptoms with IVIg at 0.4 g/kg daily for 5 days. She completed the course of IVIg and was discharged with marked improvement in left wrist extension strength (from 3-/5 to 4+/5) and resolution of tingling sensations in her feet.
The patient was scheduled to receive 1 g/Kg IVIg daily for 5 days, 28 days following discharge. A third EMG was performed 4 weeks after the initial IVIg treatment and showed the disappearance of conduction block in both radial nerves (Figure 2) but with persistent fibrillations of muscles in the left extensor indicis propius, the left brachioradialis, and the left extensor carpi radialis longus. The patient then received two courses of IVIg at 0.5 g/kg each on two consecutive days, 28 days apart. A fourth EMG revealed a neurogenic motor unit change in the left extensor indicis propius with no conduction block (Figure 3).
FOLLOW-UP AND OUTCOMES
Treatment with IVIg resulted in significant improvement of symptoms in the author’s patient, who exhibited high levels of anti-GM2 IgM antibodies, a rare association with MMN. The patient reported marked improvement in left wrist extension strength and resolution of sensory disturbances.
DISCUSSION
MMN is a rare inflammatory neuropathy characterised by progressive, asymmetric weaknessin the distal limbs without sensory loss.4 Diagnosis typically requires the presence ofconduction blocks in at least two peripheral nerves.4 In a substantial percentage of cases (30–80%), patients exhibit elevated levels of IgM anti-GM1 ganglioside antibodies, though a negative result does not exclude MMN.8
The main differential diagnoses for MMN include Lewis-Sumner syndrome (LS) and Guillain-Barré syndrome (GBS).8 LS is distinguished from MMN by the presence of significant sensory symptoms and neuropathic pain, often accompanied by diminished sensory nerve potentials.8 GBS and Fisher syndrome, while similar in some respects, can be differentiated through comprehensive laboratory and electrophysiological evaluations, as demonstrated in the authors’ case. GBS and Fisher syndrome were excluded based on normal cerebrospinal fluid protein levels without cytoalbuminologic dissociation, absence of ophthalmoplegia or ataxia, and EMG findings consistent with motor conduction blocks rather than demyelination or axonal damage. The pathogenesis of MMN suggests an autoimmune basis because of the association with anti-GM1 antibodies and robust response to immunomodulatory treatments, particularly IVIg. IVIg remains the first-line treatment for MMN based on multiple studies and randomised trials.9
While anti-GM2 antibodies have been implicated in other neuropathic conditions such as GBS and Fisher syndrome, their implication in MMN remains unclear due to limited research.10,12 Anti-GM2 antibodies are exceedingly rare in MMN, with fewer than five reported cases in the literature. This rarity underscores the uniqueness of the author’s case and highlights the need for further research into the role of anti-GM2 antibodies in immune-mediated neuropathies. Anti-GM2 antibodies have been described in patients with GBS subsequent to cytomegalovirus hepatitis,10 and in mycoplasma pneumoniae-associated acute disseminated encephalomyelitis,10 but nothing is known about their occurrence in MMN or other chronic immune-mediated neuropathies.8 The author’s case excluded GBS, LS, and Fisher syndrome by way of laboratory and electrophysiological examination. The diagnosis of MMN was supported by the characteristic clinical features, including CBs in both radial nerves, as well as the positive response to IVIg therapy. Treatment with IVIg resulted in significant improvement of symptoms in the authors’ patient, who exhibited high levels of anti-GM2 IgM antibodies, a rare association with MMN.
It is plausible that the Pfizer-BioNTech COVID-19 vaccine primed the immune system, predisposing the patient to an adverse reaction to infliximab originator. This hypothesis is supported by the temporal association between vaccination, infliximab infusion, and symptom onset. Further research is needed to explore potential interactions between vaccines and immunomodulatory therapies.
A similar case was described by Eren et al.13 involving a 52-year-old man who presented with progressive weakness in the lower extremities and gait disturbance over the course of several weeks. There was no disease or functional disability in his previous medical history. He had no history of trauma, fever, night sweats, bowel and bladder dysfunction, or infection. His symptoms began 25 days after receiving the second dose of the Pfizer-BioNTech COVID-19 vaccine.
CONCLUSION
MMN associated with anti-GM2 antibodies is a rare entity, which should be a differential in patients presenting with rapidly progressive neurological symptoms. A thorough neurological examination and testing with EMG could aid in prompt diagnosis and management with anti-GM2 antibodies where appropriate. The authors’ patient had reported improvement in symptoms after receiving treatment with IVIg. Further research is warranted to investigate the potential association between COVID-19 vaccination and the development of MMN with anti-GM2 antibodies. Additionally, studies should explore the mechanisms underlying infliximab-induced MMN to guide clinicians in managing patients with autoimmune conditions.
ETHICAL CONSIDERATIONS
Written informed consent was obtained from the patient involved in this study for the publication of their data and images.







