Abstract
EGFR mutations are the first identified targetable driver alterations in advanced non-small cell lung cancer (NSCLC), for which specific epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKI) have been developed. These small molecules, administered orally, changed the natural history of patients with EGFR-mutated NSCLC, reporting impressive response and survival data.
Osimertinib, a third-generation EGFR-TKI, can be considered the standard first-line therapy for the ‘common’ EGFR mutations, which include the exon 19 deletion and Leu858Arg point mutation in exon 21, accounting for 90% of cases. The ‘uncommon’ EGFR mutations, highly heterogeneous and with a low frequency, seem to be more sensitive to afatinib and osimertinib, a second-generation EGFR-TKI, excluding the EGFR exon 20 insertions mutations, for which a platinum-based regimen should be recommended while waiting for specific targeted inhibitors to reach the market.
However, after an initial activity to first-line EGFR-TKI treatment, a disease progression is reported due to the presence of an intrinsic resistance or the onset of an acquired resistance. The latter can be broadly grouped into EGFR-dependent or EGFR-independent mechanisms of resistance, for which several new drugs and strategic approaches are under investigation.
This review focuses on the state-of-the-art EGFR-TKIs in the treatment of metastatic NSCLC harbouring EGFR mutations, and also discusses potential future perspectives.
Key Points
1. The landscape treatment of NSCLC has dramatically changed in the last years starting from the discovery of EGFR mutations as key drivers of a subgroup of lung cancer and their inhibitors development. This led to the start of “Precision Medicine” era for this disease, an approach in continuous evolution in needs of frequent updates.2. The standard of care of EGFR-TKIs in the treatment of metastatic NSCLC harbouring EGFR mutations, the management of mechanisms of resistance, and the potential future perspectives in this setting are discussed in this article.
3. The “Precision Medicine” is a reality for the NSCLC management. The histotype and biomarkers profile of NSCLC are essential to apply the more appropriate therapeutic approach, especially in the presence of driver alterations, such as EGFR mutations, for which inhibitors are clinically available and able to change the natural history of these NSCLC subgroups.
INTRODUCTION
Epidermal growth factor receptors (EGFR), the first of four members of the ErbB family, play essential roles in both normal physiological and cancerous conditions. EGFR is characterised by an extracellular ligand-binding domain, which when activated by the ligand leads to the receptor dimerisation; a transmembrane domain, responsible for the dimerisation; and an intracellular tyrosine kinase domain that activates the intracellular kinase domain and the autophosphorylation of tyrosine residues within the cytoplasmic domain of the receptor. Through the association with intracellular signalling molecules, an EGFR is able to activate a variety of signalling pathways responsible for cell proliferation, survival, and differentiation. The function of the EGFR can be dysregulated in various types of malignancy due to gene amplification, mutations, or overexpression, becoming a promising therapeutic target.1
EGFR mutations are reported in approximately 17% and 50% of metastatic lung adenocarcinomas in people of White and Asian background, respectively.2,3 The ‘common’ mutations, that account for approximately 90% of all EGFR mutations, include the in-frame deletions around the LeuArgGluAla motif (residues 746–750) of exon 19, and the Leu858Arg (L858R) point mutation in exon 21.4 These common EGFR mutations are detected with a higher frequency in metastatic tumours in females, people from an Asian background, never-smokers, and those with lung adenocarcinoma.5 Huge radiographic and clinical responses are reported with the corresponding monotherapy tyrosine kinase inhibitors (TKI), oral small molecules that block the activation of downstream signalling induced by EGFR, by binding to the adenosine triphosphate-binding sites.4
After an initial first-line EGFR-TKI treatment activity, lasting around 10–14 months, a disease progression is reported in the majority of patients with metastatic non-small cell lung cancer (NSCLC), with mechanisms of resistance that may vary.
The ‘uncommon’ EGFR mutations account for approximately 10% of all cases, most of which are EGFR exon 20 insertion (ex20ins) mutations, and are associated with poor responses to EGFR-TKI therapy.6,7
In this article, the authors review the state of the art of EGFR-TKIs in the treatment of metastatic NSCLC harbouring EGFR mutations, and also discuss the potential future perspectives.
STATE-OF-THE-ART
EGFR mutations, especially exon 19 deletions and the L858R mutation, are associated with sensitivity to EGFR-TKIs, predicting a favourable clinical outcome for patients with NSCLC receiving this therapy.8 In fact, several EGFR-TKI generations have been developed and investigated in clinical trials, reporting results able to modify the natural history of this subgroup of patients.
First-Generation EGFR-TKIs
Gefitinib, erlotinib, and icotinib belong to the first-generation EGFR-TKIs. These small molecules are reversible inhibitors of EGFR, administered orally at the dose of 250 mg/day for gefitinib, 150 mg/day for erlotinib, and 125 mg three times per day for icotinib. First-generation EGFR-TKIs improved, with a statistical and clinical significance, the first-line outcomes of advanced NSCLC harbouring EGFR mutations when compared to platinum-based chemotherapy. The objective response rates (ORR) were 58–83% with first-generation EGFR-TKIs versus 15–45% with platinum-based regimens, with a progression-free survival (PFS) of 8.4–13.7 months versus 4.6–7.9 months, respectively. Although overall survival (OS) seemed not to improve with these small molecules, the high crossover rates mitigated the potential advantages that were reflected in both arms of each trial, reporting a range of 18.0 months to 35.5 months for EGFR-TKIs and 20.8 months to 38.8 months for chemotherapy. These survival results had never been observed in metastatic NSCLC before the introduction of EGFR-TKIs in the experimental and standard therapy of NSCLC positive for EGFR mutations (Table 1). 9-15
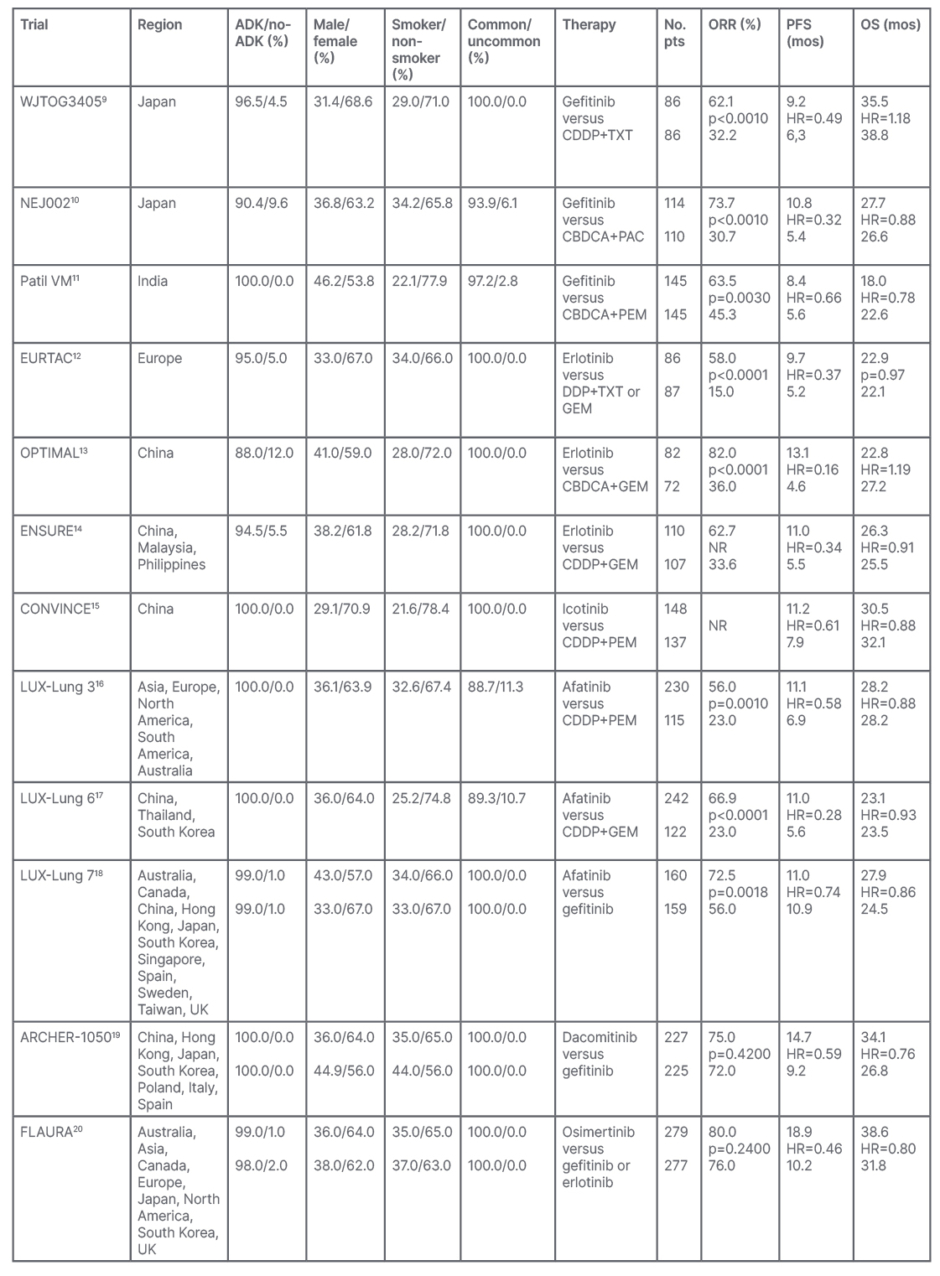
Table 1: Results of the first-line Phase III trials comparing both first- and second-generation epidermal growth factor receptors-tyrosine kinase inhibitors versus platinum-based chemotherapy, or head-to-head epidermal growth factor receptors-tyrosine kinase inhibitors, in advanced non-small cell lung cancer harbouring EGFR mutations.
ADK: lung adenocarcinoma; CBDCA: carboplatin; CDDP: cisplatin; DDP: platinum; EGFR-TKI: epidermal growth factor receptor-tyrosine kinase inhibitor: HR: hazard ratio; GEM: gemcitabine; mos: months; NSCLC: non-small cell lung cancer; No. pts: number of patients; NR: not reported; ORR: objective response rate; OS: overall survival; PAC: paclitaxel; PEM: pemetrexed; PFS: progression-free survival; TXT: docetaxel.
A meta-analysis of randomised trials that compared EGFR-TKIs to chemotherapy showed that toxic deaths were reported in 1.7% of cases, with pneumonitis being the most frequent cause, without significant differences between EGFR-TKIs. Grade ≥3 adverse events were reported in approximately 40% of patients receiving EGFR-TKIs, with diarrhoea and skin rash, typical adverse events related to this drug class, being the most frequent. In particular, the risk for increased liver enzyme levels was higher with gefitinib than with the other EGFR-TKIs. Discontinuation of treatment due to toxicity occurred in 7.7% of patients, with no significant differences between EGFR-TKIs.21 Table 2 summarises the main Grade ≥3 adverse events reported by first- or second-generation EGFR-TKIs when compared, in first-line treatment, to chemotherapy in advanced NSCLC harbouring EGFR mutations.
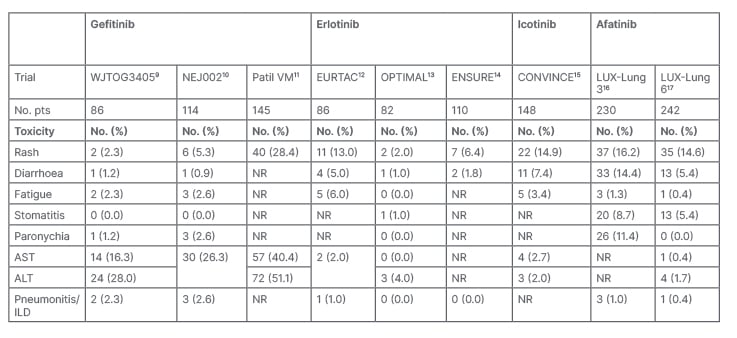
Table 2: Phase III trials main Grade ≥3 adverse events reported by first- and second-generation epidermal growth factor receptors-tyrosine kinase inhibitors when compared with chemotherapy in advanced non-small cell lung cancer harbouring EGFR mutations.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; EGFR-TKI: epidermal growth factor receptor-tyrosine kinase inhibitor; ILD: interstitial lung disease; No.: number; No. pts: number of patients; NR: not reported; NSCLC: non-small cell lung cancer.
Second-generation EGFR-TKIs
This subgroup of EGFR-TKIs includes mainly the irreversible small molecules afatinib and dacomitinib. Afatinib is administered orally at the dose of 50 mg/day, while dacomitinib is administered at the oral dose of 45 mg/day.
Afatinib was compared with platinum-based regimens in the first-line treatment of advanced NSCLC harbouring EGFR mutations, reporting statistical and clinical improvements of all outcomes. In fact, ORR was 56.0–66.9% with afatinib and 23.0% with platinum-based chemotherapy, PFS was 11.0 months versus 5.6–6.9 months and OS was 23.1–28.2 months versus 23.5–28.2 months, respectively. Furthermore, in these trials, a high crossover was reported (Table 1).16,17 The rate of Grade ≥3 adverse events for afatinib was 42.1%, with a higher risk of rash and diarrhoea for afatinib than with first-generation EGFR-TKIs (Table 2).21
LUX-Lung 7, a Phase IIb, randomised trial, compared afatinib with gefitinib in the first-line treatment of advanced NSCLC harbouring EGFR mutations. There were three co-primary endpoints: PFS, time-to-treatment failure, and OS. Despite afatinib showing a slightly better OS compared to gefitinib, these data should be interpreted with caution since they are coming from a Phase IIb study. The trial confirmed the safety profile of each drug.18
No studies compared dacomitinib to chemotherapy. Conversely, a Phase III study, ARCHER-1050, evaluated the head-to-head comparison of dacomitinib and gefitinib in first-line therapy of advanced NSCLC selected for EGFR mutations. PFS, the primary endpoint, and OS were significantly improved by dacomitinib compared to gefitinib, with adverse events typical of second- and first-generation EGFR-TKIs.19
Overall, the results of these head-to-head trials showed a superior control of disease by second-generation, irreversible EGFR-TKIs, compared to first-generation, reversible, EGFR-TKIs (Tables 1 and 3).
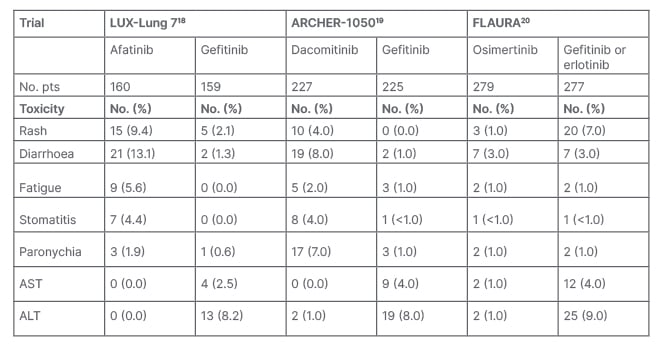
Table 3: Phase III trials main Grade ≥3 adverse events reported by Phase III trials comparing the different generation pidermal growth factor receptors-tyrosine kinase inhibitors in advanced non-small cell lung cancer harbouring EGFR mutations.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; EGFR-TKI: epidermal growth factor receptor-tyrosine kinase inhibitor; ILD: interstitial lung disease; No.: number; No. pts: number of patients; NSCLC: non-small cell lung cancer.
Third-generation EGFR-TKIs
In nearly 50% of cases, the acquired mechanisms of resistance to first- and second-generation EGFR-TKIs is due to the onset of a new EGFR mutation, the T790M, in exon 20, which causes impaired binding of the TKI.22 In order to overcome this resistance, osimertinib, a third-generation irreversible EGFR-TKI given at the daily dose of 80 mg, was developed, in view of its ability to cross the brain barrier to control central nervous system (CNS) metastases. The AURA3 Phase III, randomised trial demonstrated the superiority of osimertinib versus pemetrexed plus carboplatin or cisplatin in patients with NSCLC harbouring EGFR exon 20 p.T790M as mechanism of resistance to EGFR-TKIs.23 The AURA3 trial showed a median PFS of 10.1 months in the osimertinib arm versus 4.4 months in the chemotherapy arm (hazard ratio: 0.30; 95% confidence interval: 0.23–0.41; p<0.001) in patients without CNS metastasis. In patients with CNS involvement, the median PFS was 8.5 versus 4.2 months (hazard ratio: 0.32; 95% confidence interval 0.21–0.49), respectively.23
The FLAURA Phase III trial compared osimertinib versus gefitinib or erlotinib in first-line treatment of advanced NSCLC harbouring common EGFR mutations. Osimertinib was shown to significantly improve the survival outcomes both statistically and clinically, with a favourable safety profile (Tables 1 and 3[/h]).20 The interesting results reported in metastatic disease led to investigate osimertinib in early stages of NSCLC. The ADAURA Phase III, randomised trial enrolled Stages IB–IIIA, completely resected, EGFR mutation-positive NSCLCs to receive osimertinib versus placebo.24 The primary endpoint was disease-free survival in Stages II–IIIA, which was not reached in the osimertinib group and was 19.6 months in the placebo arm. In the overall population, the median disease-free survival was not reached in the osimertinib group, versus 27.5 months in the placebo group.24 Other third-generation EGFR-TKIs are under investigation, with imminent results.25
Uncommon EGFR Mutations
Despite the fact that common EGFR mutations are particularly sensitive to EGFR-TKIs, which represent the standard of care first-line therapy, a meta-analysis showed that exon 19 deletion is associated with longer PFS compared to exon 21 L858R mutation. This confirms that exon 19 deletions and L858R point mutations are two different disease entities in regards to outcomes of EGFR-TKIs and prognosis.26 Furthermore, this issue is also reported for osimertinib, which showed lower efficacy against EGFR L858R, with no overall benefit in the FLAURA trial.23 These considerations do not affect the standard of care first-line approach for common EGFR mutations due to the clear improved outcomes with EGFR-TKIs.
This different sensitivity is particularly true for the uncommon EGFR mutations, which are highly heterogeneous and often have very low frequencies. The most prevalent uncommon EGFR mutations include G719X, S768I, and L861Q, in exons 18, 20, and 21, respectively. These EGFR mutations are generally excluded from randomised trials and very little prospective data are available. Mainly afatinib and osimertinib, which showed a similar activity between uncommon and common EGFR mutations,might be considered for the treatment of this subgroup.27
A different group of the uncommon EGFR mutations includes the ex20ins mutations that are relatively frequent, representing approximately 4% of all EGFR-mutated NSCLCs. These mutations confer intrinsic resistance to available EGFR-TKIs, as they result in steric hindrance of the drug-binding pocket, and are usually associated with an immediate inefficacy of EGFR-TKIs.27 The first-line standard approach is a platinum-based regimen; thus, a different approach was investigated for this specific group of patients. Among the drugs under development, amivantamab is an anti-EGFR and anti-mesenchymal epithelial transition factor (MET) bispecific antibody with immune cell-directing activity, which binds to each receptor’s extracellular domain, bypassing resistance at the TKI binding site. In a total of 81 previously treated patients, amivantamab, given intravenously at the dose of 1,050 mg if the patient’s weight was <80 kg or 1,400 mg with a weight ≥80 kg, showed an ORR of 40%, with a median duration of response of 11.1 months. The median PFS was 8.3 months, with the most common Grade ≥3 adverse events being hypokalaemia in six patients (5%), and rash, pulmonary embolism, diarrhoea, and neutropenia in four (4%) each.28 These results led amivantamab to become the first treatment to be approved by the U.S. Food and Drug Administration (FDA) for patients with advanced NSCLC harbouring EGFR ex20ins mutations whose disease has progressed on or after platinum-based chemotherapy. Mobocertinib, a first-in-class EGFR-TKI being developed for the treatment of EGFR ex20ins-positive NSCLC, also granted accelerated approval by FDA, in the same setting of amivantamab, at the daily oral dose of 16o mg.29
EGFR MECHANISMS OF RESISTANCE
The immediate inefficacy of first-line EGFR-TKIs leads to consideration of an intrinsic resistance that is often due to the presence of non-sensitive EGFR mutations, such as EGFR ex20ins mutations.27 The presence of concurrent molecular or genetic alterations, which could potentially decrease the sensitivity of patients with sensitising EGFR mutations to EGFR-TKI treatment, may be considered as other agents responsible of intrinsic resistance.30
EGFR mutation-positive NSCLCs, after a variable period of response to EGFR-TKIs, develop acquired mechanisms of resistance with progression of the disease. To define the acquired resistance to first-line EGFR-TKIs, a clinical definition was proposed to benefit both practising oncologists and researchers.31 Moreover, according to the progression disease, a clinical subtyping of acquired resistance was also proposed, namely CNS only, oligo-progression, and systemic progression.32 These classifications can help physicians to define the most appropriate therapeutic approach to this group of patients according to progression patterns.33
The most frequent EGFR-dependent mechanism of resistance, for the first- and second-generation EGFR-TKIs, is the onset of a new EGFR mutation, the T790M, which is sensitive to third-generation EGFR-TKIs.25
The histological transformation from adenocarcinoma to small cell lung cancer (SCLC) was observed by rebiopsies performed during the natural history of patients with EGFR-mutated NSCLC. This phenomenon is considered another mechanism of acquired resistance to EGFR-TKIs, probably due to the origination of SCLC cells from minor pre-existent cells under the selection pressure of EGFR-TKIs, or transdifferentiated from the adenocarcinoma cells, or arisen from the multipotent stem cells.34 Once the tumour transformation in SCLC is diagnosed, the standard chemotherapy for this histotype leads to a response and survival to treatment comparable to that of classic SCLC.35,36
At progression from EGFR-TKIs, in the view of a strategy of biomarker-driven approaches, the amplification of the MET oncogene, the most frequent EGFR-independent mechanisms of resistance, accounts for 5–20% of EGFR-TKIs acquired resistance causes, regardless of the EGFR-TKI generation or line of treatment. MET, a transmembrane tyrosine kinase receptor, is activated by the hepatocyte growth factor, promoting the activation of the downstream AKT pathway, leading to cell proliferation, survival, and antiapoptosis. The most widely-adopted definition for MET amplification is the presence of the MET gene with a copy number of ≥5 or a MET/CEP7 ratio of ≥2.37 Tepotinib, capmatinib, and savolitinib are daily orally-administered potent MET inhibitors that demonstrated activity in MET exon 14 skipping mutations that occur in 3–4% of NSCLC.38-40 These MET inhibitors have also demonstrated preclinical activity in EGFR-mutant/MET-amplified models of acquired EGFR-TKI resistance when combined with the same EGFR-TKI. A Phase Ib/II study showed that the combination of capmatinib, at the dose of 400 mg twice daily, plus gefitinib at the standard dose, is a promising treatment for patients with EGFR-mutated, MET-amplified NSCLC, who experienced disease progression while receiving EGFR-TKI treatment.41 In a Phase Ib trial, the combination of osimertinib at the standard dose, and savolitinib at the weight-based dosing of 300 mg or 600 mg, showed encouraging antitumour activity in patients with MET-amplified, EGFR mutation-positive, advanced NSCLC, who had disease progression on a previous EGFR-TKI.42 Based on these results, the SAVANNAH Phase II trial of osimertinib plus savolitinib for patients with EGFR-mutant, MET-amplified NSCLC, following disease progression on osimertinib, is ongoing.43 INSIGHT 2, an international, open-label, multicentre, Phase II trial, is an ongoing study assessing the combination of tepotinib at the dose of 500 mg/day, plus osimertinib at the standard dose, in patients with EGFR-mutant NSCLC and acquired resistance to first-line osimertinib due to the onset of MET-amplification.44
The second most frequent mechanism, behind MET-amplification, of acquired resistance to first-line osimertinib is the onset of the tertiary EGFR C797S mutation, which occurs in exon 20 with a frequency of 7%.45 Several further mechanisms of acquired resistance to EGFR-TKIs have been described, with the future perspectives of defining the best strategy to overcome them.46
At progression from third-generation EGFR-TKIs, in the presence of a nontargetable biomarker or in the absence of an identified molecular target, chemotherapy with platinum-based regimens is the appropriate strategic approach.47,48
FUTURE PERSPECTIVES
Several efforts are ongoing to improve the outcomes of this subgroup of patients with NSCLC, both in overcoming the acquired resistances and in defining strategies to delay and prevent the emergence of these resistances.
In this regard, a meta-analysis pooled the results of randomised trials comparing first-generation EGFR-TKI monotherapy versus the combination of the same EGFR-TKI plus chemotherapy. The results showed that the combination therapy significantly improved ORR, and prolonged PFS and OS of first-line treatment in patients with advanced NSCLC harbouring activating EGFR mutations, especially when chemotherapy included platinum-based doublets in concurrent administration with EGFR-TKI. No differences were observed between EGFR 19 deletion and L858R, probably related to the addition of chemotherapy to the EGFR-TKI. Even though an increasing incidence of adverse events was registered in the combination arm, the treatment was tolerable and clinically manageable.49 Based on these interesting results, and the availability of third-generation osimertinib, the FLAURA-2 trial of osimertinib with or without platinum–pemetrexed regimen as first-line treatment in patients with mutated EGFR NSCLC is ongoing, with PFS as the primary endpoint.23
Fourth-generation allosteric EGFR-TKIs, such as EAI045, JBJ-04-125-02, BLU-945, CH7233163, TQB3804, and BBT-176, designed to target drug-resistant EGFR C797S and T790M mutations, are in development with in vitro and in vivo activity. These drugs are able to target contemporary common EGFR mutations, with T790M and C797S also appealing for an upfront approach. To date, several preclinical studies showed promising results, but no clinical data are available yet.33
CONCLUSION
The detection of the EGFR mutation status for metastatic lung adenocarcinoma and/or never-smokers is mandatory before defining the most appropriate therapy for each patient. Osimertinib remains the standard of care for the first-line treatment of patients with advanced common EGFR mutation-positive NSCLC Afatinib and osimertinib should be considered as the treatment of choice for uncommon EGFR mutations, excluding the ex20ins mutations, for which platinum-based regimen should be recommended while waiting for specific targeted inhibitors to reach the market.
The growing understanding of the mechanisms responsible of the progression, growth, and metastatic diffusion of lung cancer cells leads to a genomic profiling knowledge for the development of targeted therapies able to control and change the natural history of the disease since the first diagnosis. The development of next-generation sequencing platforms and targeted gene panels analyses may play an essential role in detecting early signals of drug resistance. In this way, there is the opportunity to switch to an alternative treatment to overcome relevant subclonal progression. However, this approach might be challenging due to the possible difficulty in reaching the tumour lesion, especially in patients with NSCLC, with the need of invasive and potentially harmful procedures. In this regard, the increasing development and optimisation of liquid biopsies, based on blood sampling, may noninvasively detect targetable genomic alterations, thus guiding the corresponding targeted therapy. Furthermore, liquid biopsy might be particularly useful in monitoring the response to treatment, detecting the onset of genetic changes as an early signal of resistance. All these efforts are first steps towards precision medicine, defined as the right drug to the right patient but also at the right moment.50








