Abstract
Objectives: This study assessed the effect of liraglutide as a monotherapy and add-on to metformin on weight loss and BMI, among patients with Type 2 diabetes (T2D) who are overweight or obese.
Methods: The following databases were assessed to identify relevant papers published until July 2023: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), clinicaltrial.gov, and Web of Science. All clinical trials evaluating the effect of liraglutide on weight loss and BMI in patients with T2D who are obese or overweight, treated for at least 2 months, were included in the review. All analysis and risk of bias assessment was done using Cochrane Review Manager software, version 5.4.1 (Cochrane, London, UK). A random-effects model with inverse variance was used to synthesise the results.
Results: In total, 10 randomised controlled trials involving 945 participants were included in the meta-analysis. Treatment with liraglutide with or without metformin for more than 2 months led to a significant weight loss (mean difference: -4.75 kg; 95% confidence interval: -7.02–-2.48; p<0.01). Liraglutide supplementation also led to a significant decrease in BMI (mean difference: -2.07; 95% confidence interval: -2.75–-1.39; p<0.01). However, the decrease in weight and BMI was not statistically significant as compared to treatment with other oral hypoglycaemic drugs or placebo.
Conclusion: Liraglutide used alone or as adjunctive therapy to metformin produces reduction in weight and BMI when administered in adult patients with T2D who are obese or overweight.
Key Points
1. Liraglutide, when used as a monotherapy or in combination with metformin, can lead to a significant reduction in weight and BMI in patients with Type 2 diabetes who are overweight or obese.2. The study found that liraglutide’s effect on weight loss and BMI was statistically significant compared to baseline measurements, but it was not significantly better than other oral hypoglycaemic drugs or placebo.
3. The GRADE recommendation for the evidence in this study varies from moderate certainty of evidence for BMI reduction to very low certainty evidence for weight reduction, indicating the need for further studies.
INTRODUCTION
Obesity is a major risk factor for the development of Type 2 diabetes (T2D), with the prevalence of both increasing in a parallel manner. The appearance of the term ‘diabesity’ across different articles and literature shows that many patients with diabetes are obese.1 In this subgroup of patients, the therapeutic goal is to achieve adequate blood sugar control and weight loss. Among the available pharmacotherapies for T2D, metformin and dipeptidyl peptidase-4 (DPP-4) inhibitors are shown to help with weight reduction.2
Glucagon-like peptide-1 (GLP-1) receptor agonists are recommended as initial therapies by the American Diabetes Association (ADA) based on glycaemic needs, and for individuals at high risk for atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.3 Liraglutide is a long acting GLP-1 analogue available in the market as Victoza® (1.8 mg subcutaneous injection; Novo Nordisk, Bagsværd, Denmark) and Saxenda® (3.0 mg subcutaneous injection; Novo Nordisk).4,5 Liraglutide 1.8 mg was approved by the U.S. Food and Drug Administration (FDA) for the management of T2D in adults and paediatric patients aged 10 years and older, as an adjunct therapy to diet and exercise.6 A meta-analysis comparing GLP-1 agonists (exenatide, liraglutide, albiglutide, semaglutide, lixisenatide) with placebo shows weight reduction of approximately 3% at 6 months in the GLP-1 agonists group.7 Subsequently, liraglutide 3.0 mg was approved for chronic weight management in adult patients (2014) and in patients aged 12 years or older (2020) with an initial BMI of ≥30 kg/m2 (obese) or ≥27 kg/m2 (overweight) in the presence of one or more weight-related conditions (e.g., hypertension, T2D, or dyslipidaemia).8 Another meta-analysis of adults without T2D who are obese reported significant weight loss in the group treated with GLP-1 receptor agonists (semaglutide, liraglutide).9
GLP-1 is a hormone secreted by enteroendocrine cells in response to food intake, stimulating postprandial glucose-induced insulin secretion and suppressing glucagon secretion. Besides glucose homeostasis, it delays gastric emptying and promotes satiety.10 Peripheral and central administration of GLP-1 not only inhibits food intake through its central action in the hypothalamus, but also reduces leptin-induced activation of GLP-1 in the brain. Conversely, GLP-1 antagonists have been shown to stimulate feeding in rats.11 Weight loss achieved with GLP-1 agonists appears to be related to this mechanism. GLP-1 produced in the intestine is rapidly cleaved by DPP-4 and therefore has a very short half-life (<2 minutes) in the bloodstream.12 Sitagliptin, a DPP-4 inhibitor that acts to prolong GLP-1 circulation time, has also been reported to cause weight loss in patients with T2D.13 Liraglutide, a human GLP-1 analogue with 97% homology to endogenous GLP-1, has a half-life of 13 hours and is suitable for once a day dosing.14 A meta-analysis comparing the effects of sitagliptin and liraglutide on patients with T2D who are overweight/obese shows a greater reduction in haemoglobin A1c, fasting plasma glucose, postprandial plasma glucose, and weight in the liraglutide-treated group.15
The present systematic review and meta-analysis assessed the effect of liraglutide treatment as monotherapy and add-on therapy to metformin on reduction of weight and/or BMI in adult patients with T2D who are obese or overweight.
METHODS
Eligibility Criteria
Inclusion criteria
Eligible randomised controlled trials (RCT; published in English) involving human subjects were included in the analysis if they met the following framework: patients with T2D who were overweight or obese receiving monotherapy with liraglutide/Victoza/Saxenda, or combined treatment of liraglutide with metformin for at least 2 months, providing weight and/or BMI values.
Exclusion criteria
Exclusion criteria included Type 1 diabetes; prediabetes; patients who were not overweight or obese; use of other glucose lowering therapies; mixed treatment with liraglutide; non-randomised controlled trials; reported use of weight loss agent/surgery in the intervention group; studies with treatment duration less than 2 months; and treatment with any other oral antidiabetic drugs (except for short-term treatment with insulin at the discretion of the investigator).
Outcomes
The primary outcome was post-treatment weight (at last follow-up) and secondary outcome was post-treatment BMI.
Literature Search/Information Sources
The following electronic databases were searched for related publications until July 2023: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), and Web of Science. The search syntax contained related keywords as follows: (((“Type 2 diabetes”) AND (Weight OR Obesity OR BMI OR Body Mass OR Quetelet) AND (Liraglutide OR Victoza OR Saxenda))).
Study Selection and Eligible Papers
Titles and abstracts were scanned by two reviewers independently to identify eligible studies for further assessment. Selected abstracts were screened to remove studies that did not meet the inclusion criteria. The rest were further assessed for eligibility by reviewing the full text. Final papers were selected and reviewed.
Data Collection
The following data were collected for each included study: study design, title, author’s name, year of publication, sample size, study duration, patients’ age, weight and/or BMI at baseline, and study end. All the data were extracted by one reviewer and validated by a second reviewer.
Risk of Bias and Study Quality
Two authors independently assessed the risk of bias of selected articles using version 2 of the Cochrane Risk of Bias tool (Cochrane, London, UK). Disagreements were resolved by discussion or consultation with a third author. Risk of bias for included studies was assessed using the Cochrane Review Manager 5.4.1 risk of bias assessment (Cochrane). All the articles were evaluated based on the following domains: random sequence generation, allocation concealment, participant and staff blinding, outcome assessment blinding, incomplete outcome data, and selective reporting. Articles were classified as having low, unclear, and high risks of bias in each domain. Funnel plots were made to ascertain publication bias and they had minimal asymmetry on visual inspection. The authors did not exclude any studies due to risk of bias. They also assessed GRADE-pro evidence using the validated tool.16 This review has not been registered and the review protocol has not been published.
Statistical Analysis
The analysis was performed using RevMan 5.4.1 (Cochrane). An inverse variance random effects model was used to solve for mean differences (MD) in body weight and BMI between the treatment groups. Also, 95% confidence interval (CI) and χ2 values were calculated. The I2 statistic was used to assess the within-group heterogeneity to measure the degree of variation between studies. All the statistical tests were two-tailed and p<0.05 was statistically significant for all the tests.
RESULTS
Studies were selected according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.17 A total of 645 citations were retrieved from the initial literature search of the aforementioned database. Of these, 211 were multiples, leaving 434 unique study titles or abstracts. After an initial review of abstracts and titles, 107 papers were excluded, resulting in 327 potentially relevant titles. Further evaluation resulted in removal of 317 titles, leaving 10 articles to be analysed.18-27 However, quantitative analysis could be done only for eight studies for BMI and nine for body weight studies.
The characteristics of the included studies and the pooled results of the meta-analysis are summarised in Table 1. Weekly dose titration of liraglutide was performed and patients were administered the maximum tolerated dose ranging from 1.2–1.9 mg subcutaneous once daily in the included studies. Five studies used liraglutide as monotherapy, four RCTs added liraglutide to a stable dose of metformin, and participants in one RCT received metformin plus a statin. The comparator groups received placebo or other oral hypoglycaemic agents (metformin, pioglitazone, glimepiride, and insulin glargine) alone or in combination, with or without lifestyle modification in the form of exercise.
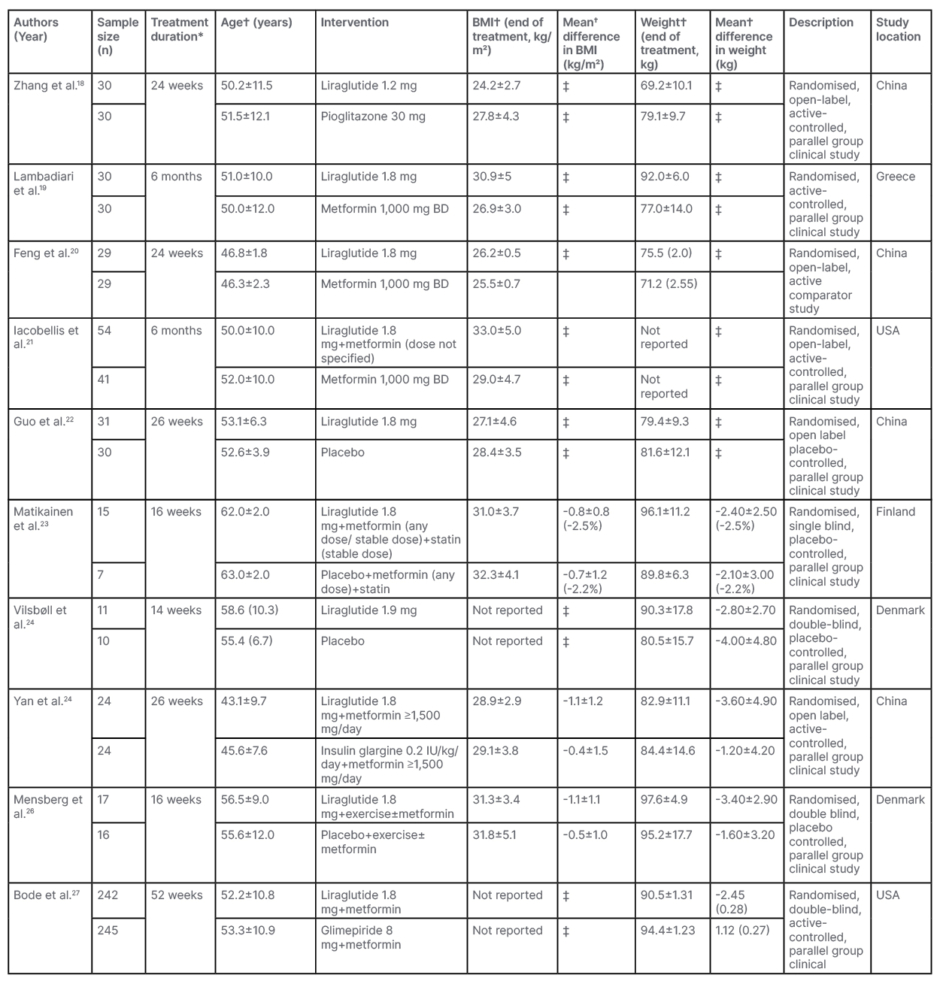
Table 1: Characteristics of included studies.
*Minimum treatment duration was 2 months for inclusion.
†Expressed as mean±standard deviation or mean (standard error) with (%) change.
‡Not calculated due to non-availability of data.
BD: twice daily.
Effect Of Liraglutide Versus Control Treatment on Body Weight
In this meta-analysis of nine RCTs with n=850, patients treated with liraglutide alone (n=131) or as an adjunct to metformin (n=298) weighed more than the control group (pioglitazone 30 mg once daily [n=30], metformin 1,000 mg twice daily [n=59], placebo±metformin [n=63], insulin glargine at starting dose 0.2 IU/kg/day+metformin [n=24], glimepiride 8 mg once daily [n=245]) after ≥2 months of treatment (MD: -1.35 kg; 95% CI: -5.75–3.04; p=0.55; Figure 1A).
Effects of Liraglutide on Body Weight (Pre- Versus Post-treatment)
Nine RCTs involving 429 participants found significant weight loss with liraglutide alone (n=131) or as an adjunct to metformin (n=298) for ≥2 months (MD: -4.75 kg; 95% CI: -7.02–-2.48; p<0.01; Figure 1B).
Effects of Control Antidiabetic Drugs on Body Weight (Pre- Versus Post-treatment)
Analysis of nine RCTs involving 421 participants indicated that control regimens (antidiabetic drugs pioglitazone 30 mg once daily, metformin 1,000 mg twice daily, placebo±metformin, insulin glargine at starting dose 0.2 IU/kg/day, glimepiride 8 mg once daily) also resulted in weight loss (MD: -0.99 kg; 95% CI: -3.59–1.62; p=0.46; Figure 1C).
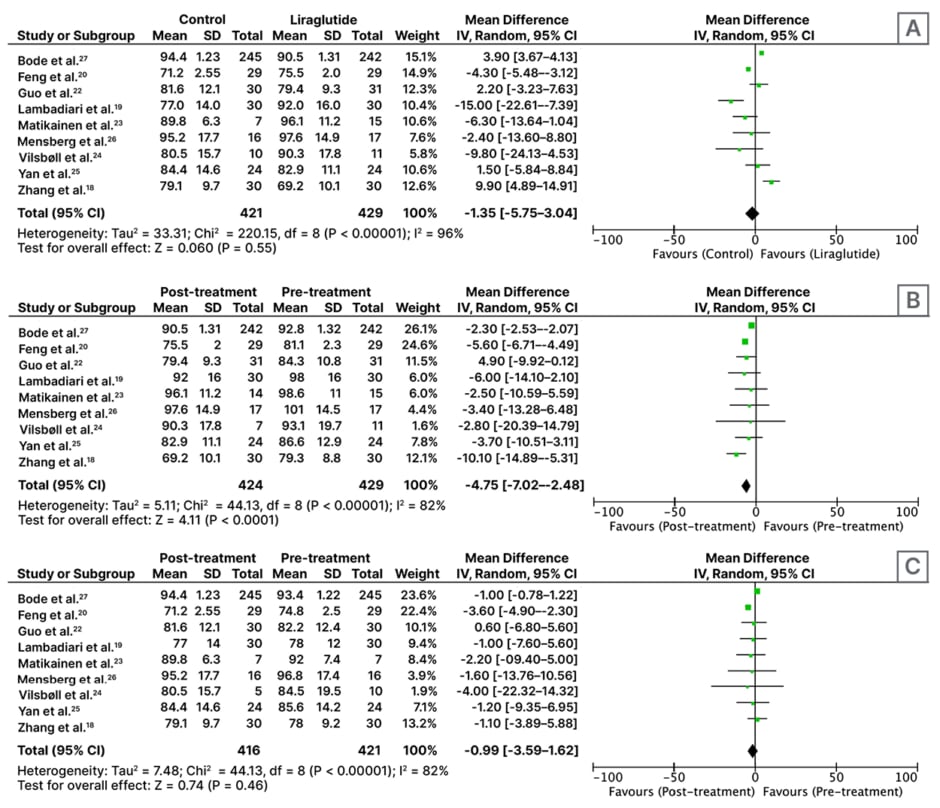
Figure 1: Forest plot for effect of A) liraglutide versus control; B) pre- versus post-liraglutide; and C) pre-versus post-control medicines on weight, following administration for at least 2 months.
CI: confidence interval; IV: inverse variance; SD: standard deviation.
Effect of Liraglutide Versus Control Treatment on BMI
This analysis of eight RCTs involving 437 participants shows that patients treated with control antidiabetic drugs (n=207) have a lower BMI as compared to patients treated with liraglutide alone (n=120) or with metformin (n=110) after ≥2 months of treatment (MD: -0.30; 95% CI: -1.82–1.22; p=0.70); however, this is not statistically significant (Figure 2A).
Effects of Liraglutide on BMI (Pre- Versus Post-treatment)
An analysis of eight RCTs involving 230 participants found that treatment with liraglutide for ≥2 months resulted in a significant reduction in BMI (MD: -2.07; 95% CI: -2.75–-1.39; p<0.01; Figure 2B).
Effects of Control Antidiabetic Drugs on BMI (Pre- Versus Post-treatment)
Analysis of eight RCTs with n=207 participants found that control antidiabetic drugs (pioglitazone 30 mg once daily [n=30], metformin 1,000 mg twice daily [n=102], placebo±metformin [n=55], insulin glargine starting dose 0.2 IU/kg/day+metformin [n=24]) resulted in a significant reduction in BMI (MD: -0.98; 95% CI: -1.65–-0.35; p<0.01; Figure 2C).
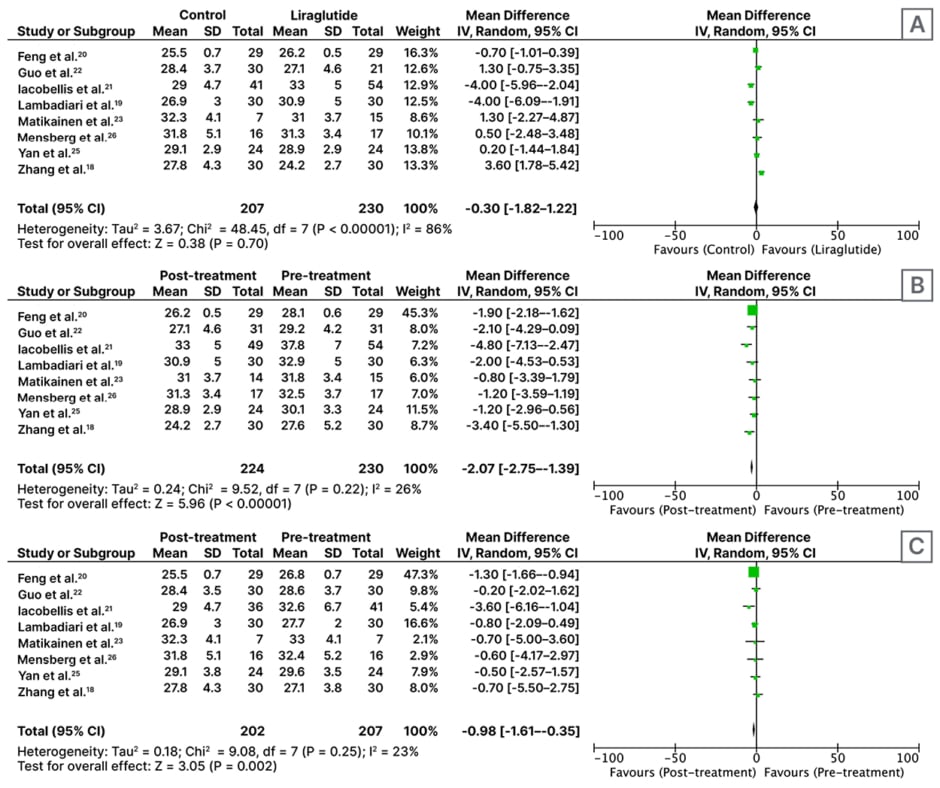
Figure 2: Forest plot for effect of A) liraglutide versus control; B) pre- versus post-liraglutide; and C) pre-versus post-control medicines on BMI, following administration for at least 2 months.
CI: confidence interval; IV: inverse variance; SD: standard deviation.
GRADE Recommendations for the Evidence
Following treatment with liraglutide, the GRADE recommendation for BMI was rated as moderate certainty of evidence, and very low certainty of evidence for weight reduction as the I2 statistic was higher. When comparing the effect of liraglutide versus control, GRADE evidence was very low for both weight and BMI reduction.
DISCUSSION
The main finding of this meta-analysis, which included 10 RCTs and compared data from 945 participants, was that liraglutide at doses of 1.2–1.9 mg once daily, produced reduction in weight and BMI when administered in adult patients with T2D who were obese or overweight. In these patients, liraglutide was used as a monotherapy or as an adjunct therapy to other oral glucose-lowering drugs. However, there was no significant effect of liraglutide when it was compared to the control treatment, and in effect it appeared less effective in causing weight loss (Figure 1A).
Beneficial effects of liraglutide on glycaemic control parameters, including glycated haemoglobin, fasting glucose, and fasting insulin levels, are well documented. Several studies using varying doses of liraglutide (0.6–3.0 mg) as monotherapy or added to other antidiabetic therapy, showed reduced body weight, improved glycaemic and metabolic control, reduced fat mass and fat percentage, and alteration in central nervous system response to food, in a wide variety of patients regardless of body weight or T2D status, including patients with schizophrenia spectrum disorder being treated with atypical antipsychotics.28-33
Thus, the authors’ results are consistent with those of the aforementioned study, but the patient population in the authors’ meta-analysis was different, i.e., they had T2D and the treatment period was much shorter.
Mechanistic studies were conducted to investigate whether GLP-1-related weight loss in humans was associated with changes in hormone levels important in energy homeostasis using the maximum daily dose (1.8 mg) of liraglutide. An earlier study found that liraglutide treatment did not affect 24-hour energy expenditure (12.6 kJ/h) in comparison to placebo (13.7 kJ/h; p=0.799).34
GLP-1 has also been suggested to suppress energy intake in humans.35 It is estimated that 80% of people with T2D are obese, and losing weight is beneficial as even more people may be overweight due to accumulation of abdominal fat. It would be desirable if peripherally administered GLP-1 induced weight loss by reducing energy intake. A major drawback is that native GLP-1 has a very short plasma half-life due to rapid degradation by intravenous dipeptidyl-peptidase (t0.5=1.5 minutes after intravenous injection). Liraglutide has a prolonged action (t0.5=13 hours) and is suitable for once-daily dosing.
These results appear similar to the authors’ analysis. It is understood that reductions in visceral and abdominal subcutaneous adipose tissue are associated with improvements in fasting glucose levels, glucose tolerance, and lipid profiles, and would be especially valuable.36
GLP-1 agonists improve glycaemic control by stimulating insulin release, and are approved for T2D. Due to their delayed gastric emptying and promoting satiety effects, they also have roles in decreasing body weight. Based on evidences from RCTs showing reduced body weight and improved metabolic control, liraglutide 3 mg daily subcutaneous was approved for adult patients with obesity in 2014, which was then extended for adolescents aged 12–17 years in 2020.28 Semaglutide (2.4 mg once weekly) was then approved in 2021 for weight control in adults who are obese or overweight with weight-related complications.37 Semaglutide and liraglutide 1.8 mg doses have also been shown to reduce the risks of serious cardiovascular events in patients with T2D.38,39 There are no data on the effect of liraglutide 3 mg on cardiovascular outcomes.40 Tirzepatide, a dual GLP-1 and glucose-dependent insulinotropic peptide agonist, received fast-tracked designation from the FDA in 2022.41 In the SURMOUNT-1 study, tirzepatide 5 mg, 10 mg, and 15 mg resulted in a weight loss of 5% or more in 85%, 89%, and 91%, respectively, over the 72 weeks study period. The most common side effect was gastrointestinal disturbance.42
Metformin used as a first-line treatment for patients with T2D, has not been approved as a treatment for obesity, but metformin used for the treatment of T2D can cause weight loss.43 The ADA recommends metformin for patients who are obese and are at high risk of developing T2D.44 As compared to anti-obesity drugs approved by the FDA, it has a relatively milder side effects profile, the most common being gastrointestinal reaction, which usually decreases with long-term use. It also has added advantages of decreasing low-density lipoprotein cholesterol level by improving lipid metabolism and alleviating symptoms of hyperandrogenism in females suffering from polycystic ovary syndrome.45,46 A meta-analysis of metformin treatment in different populations concluded that metformin treatment leads to approximately one unit reduction in BMI after 3–32 months of treatment, making it a good option for patients who are overweight or obese and at high risk for diabetes.47 Pioglitazone has been reported to cause increase in body weight and BMI in several studies and meta-analyses. However, due to its favourable effects on major adverse cardiovascular events and lipid profile, it is still being prescribed by many clinicians.48,49 When combined with metformin, the slight increase in weight is accompanied by improved glycaemic control.50 Glimepiride is a second generation sulfonylurea, usually prescribed as a second line agent for T2D. Although increase in weight is reported when compared with DPP-4 inhibitors, it is unlikely to be clinically relevant.51 Conversely, a decrease in weight was reported when glimepiride was combined with metformin in another trial.52 Use of metformin by a large number of patients in the control group (n=146 of 421 for weight and 181 of 211 for BMI) might explain why there was no statistically significant reduction in weight and BMI in the liraglutide-treated group as compared to the control group.
This meta-analysis has several limitations, the first being lack of sufficient studies, which limits the overall sample size. Second limitation is the short duration of study period. Although the authors kept a minimum of 2 months duration as an inclusion criterion, this also may be insufficient to make clinically meaningful conclusions for conditions like obesity and overweight. Substantial heterogeneity in the included studies, as well as mostly unclear risk of bias, are other limitations. For these reasons, the GRADE recommendation is moderate to very low for most outcomes.
To conclude, results of this meta-analysis suggest that liraglutide alone or as add-on treatment to metformin produces reduction in weight and BMI when administered in adult patients with T2D who are obese or overweight. However, further well-controlled studies with larger sample size and longer treatment duration are warranted to strengthen these initial findings.







