Abstract
Osteoarthritis (OA) is a chronic condition that can lead to pain, disability, and loss of function. There are currently few pharmacological treatments, and none are disease modifying. It is important to identify new treatments to reduce associated morbidity, as well as high costs to the individual and society. OA pathogenesis involves the cartilage, synovium, and bone, with many mediators in the immune system implicated in this process. These provide several targets for pharmacotherapy that can be divided into treatments that target pain or disease-modifying drugs that aim to maintain the joint. Previous OA trials conducted have failed to either meet efficacy or safety measures. Notably, anti-nerve growth factor drugs that were superior to placebo had several adverse events that subsequently limited their usage. The aim of this paper is to highlight the current treatments under investigation that are in Phase II and III of development.
This review searched trials that have been registered on clinicaltrials.gov with the term “osteoarthritis” and a primary completion date of 2021 or after that are in Phase II and III. This identified 252 studies, 52 of which were included in the review after screening and eligibility checks, that were then categorised as either targeting pain and inflammatory pathways or disease-modifying osteoarthritis drugs. Two further papers were included as they present two distinct therapies for hand OA. The results showed numerous avenues in development, with promising results, and provides hope to reduce the global burden of morbidity. It is important that these treatments are affordable for this chronic condition.
Key Points
1. Osteoarthritis (OA) is a common arthritic condition, affecting 10 million people in the UK alone. There are currently no effective disease-modifying anti-rheumatic drugs licensed for the treatment of OA.2. This study was a narrative review of clinical trials identified from clinical trials databases to identify Phase II and III studies with a primary completion on or after December 2021. The authors identified 52 studies categorised as either targeting pain modulation /inflammatory pathways or disease-modifying OA drugs that were reviewed in this article.
3. There is a strong pipeline of drug discovery with Phase II and III trials of new therapeutic agents in OA that are targeting several pathways including inflammation, pain, and cartilage regeneration.
INTRODUCTION
Osteoarthritis (OA) is the most common arthritis worldwide, causing pain, disability, and loss of function due to chronic joint disease.1 The most affected joint is the knee, followed by the hand and hip.1 Knee OA makes up 85% of the clinical burden of disease.2 There is a higher prevalence of radiologically evidenced OA compared to symptomatic OA.2 Significant costs are associated with the disease, including direct costs such as medication and surgery, but also indirect costs due to early retirement and loss of work.2 There remains a huge unmet need to develop new treatments for OA. This narrative review will discuss the pathogenesis of OA, current management, and clinical trials exploring new treatment avenues.
Pathogenesis
OA is an active disease in which there is an imbalance of repair and destruction of the whole joint.2 It involves all structures, including hyaline articular cartilage, subchondral bone, capsule, synovium, and periarticular muscles.2 It is theorised there are numerous subtypes, such as neuropathic, inflammatory, and metabolic, which allow for different treatment targets.3
In the early stages of damaged cartilage repair, increased chondrocyte activity is apparent. By-products of this increased activity include matrix degradation compounds and pro-inflammatory mediators, which lead to dysregulation of chondrocyte function. These by-products affect the adjacent synovium leading to tissue hypertrophy and increased vascularity.4 There is a predominance of macrophages in the synovitis seen in OA, reflecting a more focused innate immune response compared to rheumatoid arthritis.5 The effects on subchondral bone lead to increased bone turnover and subsequent vascular proliferation invades the cartilage. The development of subchondral bone marrow lesions (BML) is observed.2 BMLs can be best visualised on T2-weighted, fat-saturated MRIs as hyperdense diffuse lesions. There is a consensus now that the presence and frequency of BMLs correlate with pain.6 The abnormal loading leads to damage and remodelling, evidenced on histological analysis by subchondral bone trabecular thickening, cysts, angiogenesis, and new nerve formation.7
Degradation of cartilage is caused by IL-1β, which binds to synoviocytes, leading to the production of matrix metalloproteinases, cyclooxygenase-2, prostaglandin E2, and nitric oxide. This accelerates degradation via nuclear factor kappa-B and MAPK signalling pathways. Cartilage destruction is further enhanced by IL-1β triggering downstream gene expression of proteolytic enzymes including the disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), also known as aggrecanases. TNF-α also plays a role in coordinating an inflammatory response and release of matrix metalloproteinases. Hence, there are many pro-inflammatory cytokines involved in OA pathogenesis that could be potential targets for novel treatments.
Current Treatment Therapies
There is good consensus on management strategies from stakeholders.8,9 First-line treatment is non-pharmacological. This includes education, exercise, weight loss, and walking aids. Encouragement of self-management is important; patient outcomes improve if interventions around non-pharmacological measures are used.10 Key messages to communicate to people with OA are about treatment approaches, the disease pathophysiology, and imaging.11 One important message is the importance of a personalised exercise plan, best delivered by a therapist, as they can consider the complexities of a patient’s situation. Two systematic reviews found exercise reduces pain, improves physical function, and improves quality of life.12,13 Another systematic review found no radiographic progression of disease or increase in pain in elderly patients who undertake low-impact exercise.14
Topical nonsteroidal anti-inflammatory drugs (NSAID) are recommended. A systematic review of 43 studies found NSAIDs superior to a placebo, with a 0.3 effect size for relieving pain and 0.35 for improving function. In the study and general population, no serious gastrointestinal and renal side effects were reported.15
Paracetamol is no longer seen as first-line after it was found to be only minimally effective when compared to placebo, while still having an adverse effect profile.16 While paracetamol can be useful in clinical practice, it should be used carefully to avoid unwanted side-effects. Oral NSAIDs are effective in managing pain; however, they have significant cardiovascular and renal side effects that need to be noted in certain patient groups. Due to these side effects, NSAIDs should be started after medical advice and for limited time periods. Guidelines suggest using concurrent proton-pump inhibitors or cyclooxygenase-2 inhibitors in appropriate patients without co-existing conditions.17
Duloxetine has been found to be efficacious in the treatment of OA.18 Centrally acting analgesics, such as nortriptyline, were compared to a placebo in a randomised control trial (RCT) in New Zealand, where it was found to have no benefit at 14 weeks for reducing pain.19 Intra-articular steroids are used for short term pain relief and as an adjunct therapy. However, regular injections are not recommended as it is associated with a greater loss of cartilage volume when compared to placebo, which was shown in a 2-year study using triamcinolone.20 Current guidelines do not favour the repeated use of corticosteroid injections for OA.
Recent Failed Treatment Targets
Many theoretical treatments have been tried and tested, with a failure to translate into clinical efficacy from animal models.21 A meta-analysis by Singh et al.,22 looked at nine studies that used colchicine in RCTs for hand and knee OA and found there was no significant improvement in pain or function.
Nerve growth factor (NGF) is implicated in pain modulation in OA.23 NGF enhances neurotropic and nociceptive pathways by interacting with its receptor, tropomyosin receptor kinase A (TrkA), modulating the pain pathway. Due to its role in the mediation of pain, pain therapies using monoclonal antibodies have been developed (e.g. tanezumab, fulranumab, and fasinumab). Tanezumab was tested in a Phase III trial and was shown as superior compared to a placebo.23 The safety profile of NGF agents has been questioned, as a dose-dependent side effect is the development of rapidly progressive osteoarthritis (RPOA).24,25 This has been related to the co-prescription of NSAIDs.24,25 One possible hypothesis for this side effect is that reduction in pain leads to increased weight bearing, causing increased tissue damage to the joint. Concerns over RPOA have led to NGF-neutralising antibodies’ use being limited to those with palliative diagnoses.26
TrkA inhibitors are also being investigated due to their role in the same pathway. Despite promising pre-clinical trials, a Phase II trial using an oral TrkA inhibitor failed to meet its primary endpoint of an improvement of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale.27 A proof-of-concept study found an intra-articular injection of a TrkA inhibitor to be effective in significantly reducing WOMAC scores at 4 weeks; however, at 12 weeks the difference was no longer statistically significant. Comparably, intra-articular steroid injections demonstrated joint pain reduction for a maximum of usually 3 weeks.28 Although, 67.3% of those who received the treatment had injection joint inflammatory reactions. The paper proposes that in the clinical setting this could be masked or ameliorated by co-prescription of other analgesic medication.28 Unlike NGF inhibitors, no damage to the joints or evidence of RPOA was noted at the end of the trial.28
Since there are currently no disease-modifying drugs available to treat OA, there has been a large growth in clinical trials investigating novel therapeutics for OA, which will be discussed further in this review.
METHODS
The authors conducted an electronic search of clinicaltrials.gov to identify clinical trials. It was chosen as the most comprehensive database for clinical trials. This was a structured search, limited to trials in Phase II and III and with a completion date on or after December 2021 to keep the review current. Initially, studies that were not regarding osteoarthritis and its treatment were excluded. A focus was put on pharmacological trials, and trials that were addressing gene therapy/mesenchymal stem cells were removed as there was a limited word count and this could be covered in a future larger review. The exclusion criteria also included existing therapies, as they are well understood. The authors sought to describe the compounds’ mechanism of action and target receptors. Where possible they discussed the merits of the trials, the foundation on which the trial was chosen (previous Phase I data).
The search term “osteoarthritis” was used and all Phase II and III trials with a primary completion on or after December 2021 were included. They identified 252 studies in total. Trials that were terminated, withdrawn, or whose status was unknown were excluded. Trials that were beyond the scope of this review were gene therapy, mesenchymal stem cells, and surgery trials, which were excluded. They also excluded trials of non-pharmacological management of OA. The 52 remaining studies are discussed in this study. Important trials that were published during this review but not available on clinicaltrials.gov are also discussed (Figure 1).
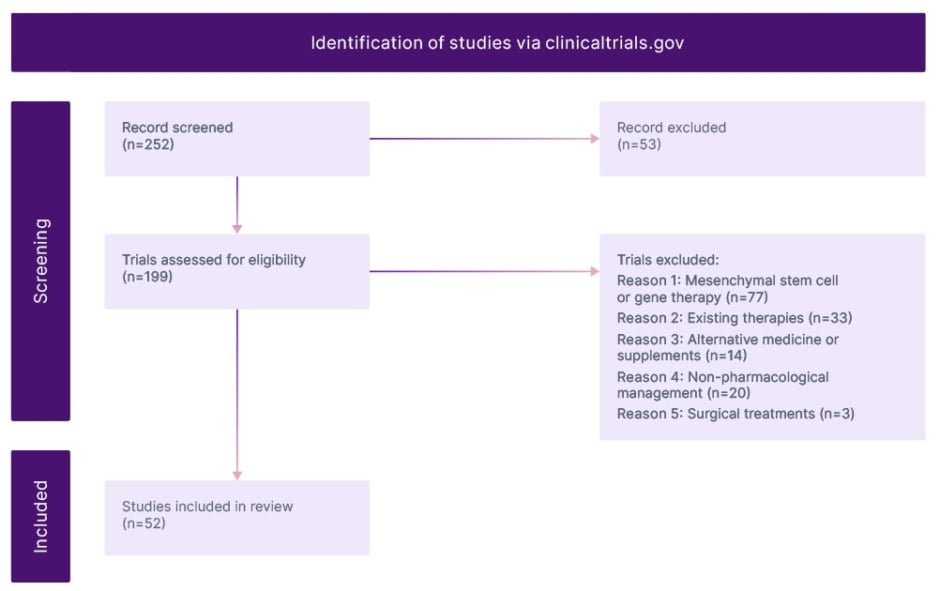
Figure 1: Modified prisma flow diagram.
RESULTS
The search yielded 52 studies, which were categorised as either targeting pain modulation/inflammatory pathways or disease-modifying osteoarthritis drugs. They are summarised in Table 1 with their mechanism of action and drug names.29-80
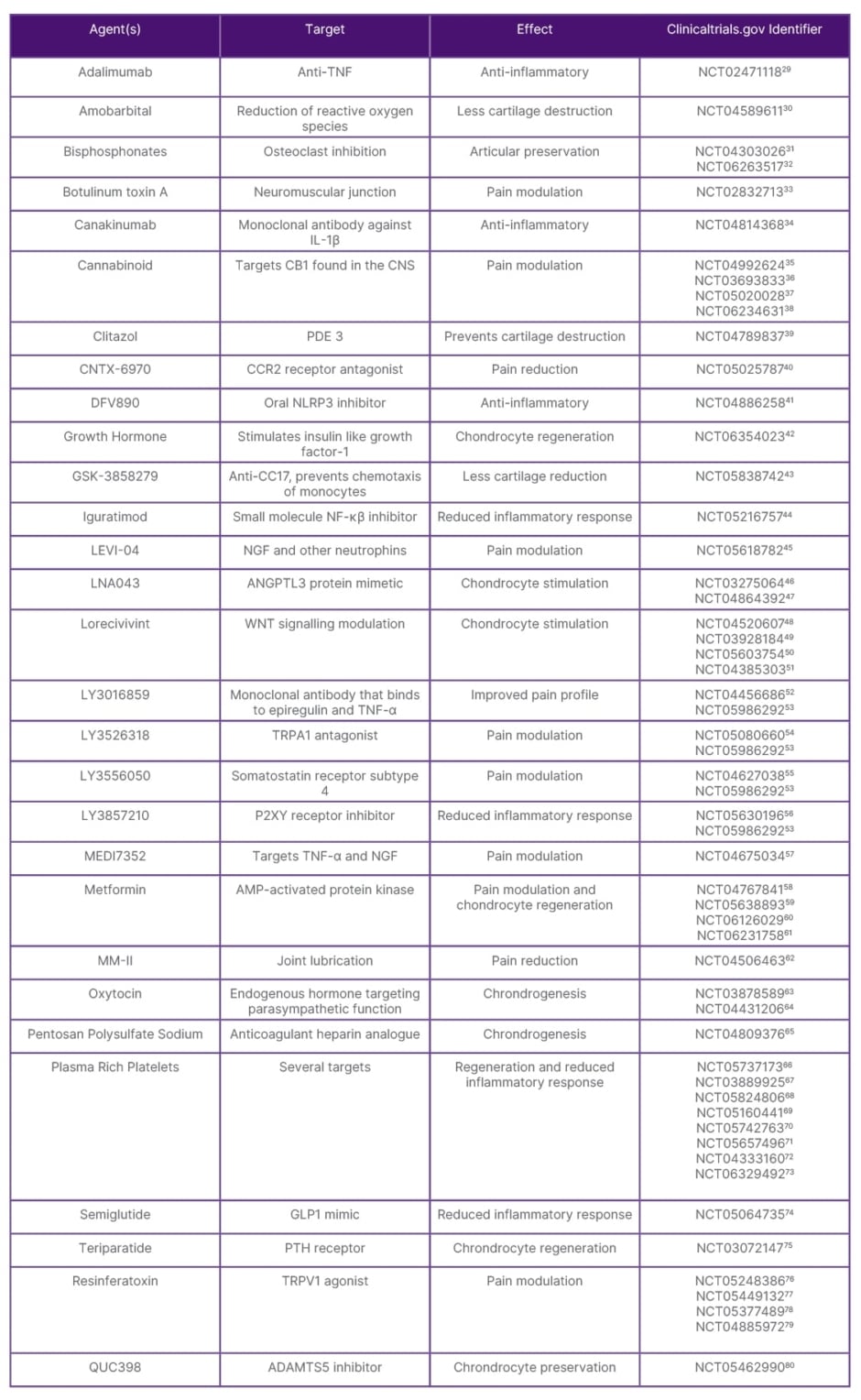
Table 1: Summary of potential therapies under investigation with a completion date in 2021 or later.
ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; AMP: adenosine monophosphate; ANGPTL3: angiopoietin-like 3; CCR2: chemokine receptor 2; CNS: central nervous system; GLP1: glucagon-like peptide 1; NF-κβ: nuclear factor kappa-B; NLRP3: pyrin domain-containing protein 3; PDE: phosphodiesterase; PTH: parathyroid hormone; TRPA1: transient receptor potential cation channel, subfamily A, member 1; TRPV1: transient receptor potential vanilloid subtype.
Treatments Targeting Pain and Inflammation
There are nociceptive pain receptors in the synovium and joint capsule but not in the cartilage. Pain sensitisation is indicated in OA joints, showing evidence of plasticity, as in normal ageing joints the number of nociceptive receptors should decrease. Pain pressure thresholds are decreased in people with OA, indicating central sensitisation.81-83
Botulinum Toxin Type A has a role in pain modulation. A systematic review in 2019 showed that at 4 and 8 weeks there are benefits compared to placebo in Visual Analogue Scale (VAS) and WOMAC.84 A current trial is looking to check for superiority over hyaluronic acid.33
Transient receptor potential vanilloid subtype 1 antagonists and modulators have been seen as a potential target of therapy. Capsaicin can induce pain desensitisation of peripheral nociceptors after an initial transient increase in pain. Low pH and heat can also activate the channel. Previously several trials were terminated due to adverse effects associated with hypothermia and impaired noxious heat sensation.85 There are current trials with Resiniferatoxin in progress (see Table 1).
Similarly, transient receptor potential cation channel, subfamily A, member 1 is involved in pain sensitisation, triggered by temperature and noxious stimuli. A recent study testing of the drug LY3526318 in patients with osteoarthritis did not find a statistically significant difference from placebo.54
LEVI-04 is a fusion protein in which the Gc portion of human immunoglobulin is fused with the neurotrophin receptor, currently in OA trials.86 It works by binding to the excess neurotrophins, unlike the anti-NGF that blocks all NGF; therefore, it is hoped that it will not produce the same side effects.
In OA pathogenesis, there is an increase of reactive oxygen species from mitochondria due to cartilage destruction. This has proven a good target for preventing post-traumatic OA in mouse models. Amobarbital blocks mitochondrial ETC member complex I and is now being investigated as an intra-articular injection in humans.30,96
There are two current trials looking at using angiopoietin-like 3 signalling pathways that work by promoting chondrogenesis.46,47 This following a successful Phase I trial showed it preserves and regenerates cartilage in vivo in OA models and it met its primary safety endpoint.97 Chemokine ligand CCL1 7 is a T cell chemokine. Elevated levels in OA are associated with elevated pain pressure thresholds.98 As well T cell interactions, it is known to affect granulocyte-macrophage colony-stimulating factor. Animal studies have shown a lack of OA pain in CCL17 gene-deficient mice.99 The role of CCL17 in OA is not fully understood. After successful Phase I trials with anti-CCL17, there is a current Phase II trial underway to find the optimum dose for pain management.99
An IL-1 inhibitor, canakinumab, a monoclonal antibody, is in Phase II trial.34 Despite being found in small amounts in synovial fluid, posthoc analysis of the CANTOS trial showed a reduction in joint replacements of those patients on canakinumab.100 There was no difference in WOMAC pain scores of VAS in a Phase II trial of intra-articular canakinumab.101 However, a current Phase II trial is testing LNA043 and canakinumab. LNA043 is a modified version of angiopoietin-like 3, which is discussed above.
Macrophages play a role in chronic inflammation, with chemokine receptor 2 playing a major role in the migration and recruitment of monocytes into tissues.102 To target this inflammatory signalling process a small molecule antagonist, CNTX-6970, is in a Phase II trial.60 Animal models have shown a reduction in pain.102,103
The NOD-Like receptor family, pyrin domain-containing protein 3 (NLRP3) inflammasome pathway leads to the reduction of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, that result in joint destruction and pain.104 Targeting this with an oral small molecule called DPV890 that binds and locks the pyrin domain-containing protein 3 inflammasome. It has been tested for various other conditions, including COVID-19, and there is a current Phase II clinical trial in patients with knee OA.41,104
Human growth hormone intra-articular injections stimulate the proliferation of chondrocytes and Type 2 collagen.105 A Phase II trial is testing growth hormone injection and will assess response to pain and cartilage regeneration.42
MEDI7352 is a monoclonal antibody which targets TNF-α and NGF, reducing the inflammatory cascade. It continues to be studied in a Phase II trial despite the halting of anti-NGF trials, which target similar pathways.57
A novel drug named MM-II, a suspension of empty large multilamellar liposomes composed of dimyristoylphosphatidylcholine and dipalmitoylphosphatidylcholine, is given as an intra-articular injection and works by providing lubrication in the joint. The results of a recent Phase IIb trial did not show a persistent statistically significant result in the reduction of WOMAC pain assessment; however, there were nominally differences in pain scores of 1 mL and 3 mL preparations and it was well tolerated.106
Oxytocin is an endogenous hormone that plays a role in bone metabolism via osteoblastogenesis and chrondrogenesis. Low levels of oxytocin have been shown in patient with osteoarthritis compared to those without in an in vitro study; there are two ongoing clinical trials to understand this process.63,64,107
Semiglutide has revolutionised Type 2 diabetes and weight loss management. It decreases blood glucose, promotes satiety, and reduces hunger by mimicking glucagon-like peptide 1. A current trial, STEP-9, will compare Semiglutide against a placebo and measure their WOMAC pain scores and weight.74,108
Another avenue of pain relief is targeting somatostatin receptor 4, which is involved in pain pathways and not involved in the endocrine actions of somatostatin. Immune cells and neurons are directly inhibited.109 This novel target is being investigated in a Phase II trial.55
Teriparatide is a synthetic form of parathyroid hormone with 34 active amino acids and is used in the treatment of osteoporosis for its role in calcium homeostasis. It has a potential role in chondrocyte regeneration, which is not well understood. Furthermore, it leads to denervation in subchondral bone, reducing pain.110 There is a current Phase II trial comparing it to placebo.75
Disease-Modifying Treatments
A meta-analysis found that bisphosphonates, a class of drugs known to inhibit osteoclast action, showed limited efficacy for pain relief in OA.111 However, there are two current trials looking at intravenous zolendronate or intra-articular clondronate.31,32
WNT signalling is part of cellular homeostasis, it determines osteoblast and chondrocyte lineage specification. Lorecivivint is an intra-articular agent that is a potential disease-modifying osteoarthritis drug.112 It modulates the WNT signally pathway by inhibiting intranuclear kinases CDC like kinase 2 and dual specificity tyrosine phosphorylation kinase 1A. In pre-clinical trials, this was effective in reducing inflammation, enhancing chondrogenesis and chondrocyte function.113 A recent Phase IIa, however, did not meet its primary endpoint of improvement in the WOMAC subscale for pain at Week 13.112 An ongoing Phase III trial has shown continued safety of the medication and an improved WOMAC pain and function scores at 12 months; despite this, at 18 months post-injection there is a loss of this effect.114
Phosphodiesterase inhibitors stop nitric oxide-mediated chondrocyte damage.115 Clitazol, normally used for the treatment of peripheral vascular disease, is a selective Type 3 phosphodiesterase, stopping the breakdown of cyclic adenosine monophosphate (AMP) and shown to prevent cartilage destruction in rats and human cartilage samples.115,116 There is a current proof-of-concept trial comparing this against conventional therapy in Egypt.117
An endpoint in OA is bone sclerosis as subchondral bone undergoes changes. Cathepsin K is primarily expressed in osteoclasts, chondrocytes, and synoviocytes, which is a cysteine protease that is involved in bone resorption.118,119 In mice with no cathepsin K, there is a high bone mass and lack of bone resorption. MIV-711 was identified as showing beneficial effects in OA and is a selective inhibitor.119 A recent RCT showed cathepsin K inhibitor had no effect on improved pain scores, but significantly reduced bone and cartilage progression.120
A potential target is ADAMTS-5 as it breaks down aggrecan. After successful Phase I trials, a Phase II trial of GLPG1972 did not meet the primary endpoint of a substantial change in the cartilage thickness compared to placebo.121 However, there are other trials currently underway with a different inhibitor, M6495, has already shown it can work ex vivo with bovine and human tissue, preserving cartilage.122 It had a successful Phase I trial showing its safety in humans, and there is now scope for further research in this area.123 There is a Phase II trial of QUC398 an intra-articular injection, targeting ADAMTS-5, which in mouse studies showed when targeted there is a reduction in pain and cartilage degeneration.79,124 A previous study of an ADAMTS-5 inhibitor failed at the Phase II stage.1 25
TPX-100 is a synthetic fragment of extracellular matrix phosphoglycoprotein, which is downregulated in OA. In animal studies, it causes articular cartilage proliferation. A Phase II clinical trial of intra-articular injection of TPX-100 found it reduced pain at 4 weeks but did not reach significance in improving patellar cartilage structure.126 It was well tolerated and there was a reduction in analgesic use. Post-hoc analysis, looking at those with moderate-to-severe bilateral tibiofemoral OA, revealed there was slower femoral bone shape change at 6 and 12 months.127
Plasma rich platelets (PRP) are an autologous blood product with a rich amount of growth factors, which has anti-inflammatory properties and reduces pain.128-130 IL-1β, TNF-α, and fibroblast growth factor are some of the catabolic cytokines that are inhibited. A systematic review comparing PRP to hyaluronic acid found it didn’t improve function but reduced pain.131 The review had issues with heterogeneity in and between studies.131 There are variations in how PRP is produced between different studies, which could account for the differences seen in efficacy outcomes.128,131 Possible variations included the amount of blood drawn, centrifuge speeds, and time. Issues with blinding in studies meant there was a possibility of a strong placebo effect.131,132 It is currently not recommended in guidelines.8,9,128
Iguratimod is a small molecule that protects the joint through immunomodulation. It inhibits NF-κβ activation, which prevents the release of inflammatory cytokines. It is used in Japan, and a current trial are looking into its effectiveness in hand OA.44
There are several clinical trials investigating metformin (Table 1). Its main action is mediated via the 5’ AMP-activated protein kinase. It leads to cartilage preservation, immunomodulation, and reduction in pain. Previous human trials were of lower quality and high risk of bias.133
Important Emerging Therapies Beyond This Search
A randomised, double-blind, placebo-controlled trial in hand OA in participants with evidence of MRI-evident synovitis showed methotrexate 20 mg once weekly had positive results. There was a moderate effect size of 0.45 at 6 months, and no effect was seen until 3 months in keeping with drug-effect in inflammatory arthritis.134 Future research could identify whether this effect persists beyond 6 months. Denosumab is anti-resorptive medication used in osteoporosis, it works by targeting RANK ligand and stopping it from activating RANK receptor found on osteoclasts. A recent Phase IIa trial of denosumab in participants with erosive hand OA showed it prevented progression of the disease.135 However, a previous study of denosumab use in knee OA showed it was not useful in symptoms or structure, although the studies had different endpoints, with the latter focusing on BMLs as a marker of progression.136
CONCLUSION
Various approaches are currently in progress to address the gap in effective therapies for OA. These range from cartilage regeneration to suppression of localised inflammation that activates inflammatory pathways inducing tissue breakdown. Since the primary symptom of OA is pain, many studies are focusing on the modulation of pain. Any treatments which are developed will need to be safe, efficacious, and cost-effective in managing this chronic condition. The enormous prevalence and chronicity mean treatments need to be inexpensive to not burden health systems. This could focus on pain modulation and structural modification in OA that are distinct treatment targets and how research into therapeutics needs to address both aspects.







