Abstract
Alzheimer’s disease (AD) is the most common form of progressively disabling degenerative dementia. In dementia in general, there are, unfortunately, even from the earliest stages of the disease, associated behavioural and psychological symptoms of dementia (BPSD), and even more pronouncedly in AD, in addition to cognitive impairment. There are no specific drugs for the treatment of BPSDs. Therefore, there remains an unmet medical need. To date, despite side effects, benzodiazepines, anti-psychotics, mood stabilisers, and anti-depressants continue to be used in the clinic. The aim of this research work is to provide an understanding of the role of benzodiazepines when used for the treatment of BPSD and cognitive impairment in AD.
Key points
1. This article is a clinical review centred on behavioural and psychological symptoms of dementia and their treatment with benzodiazepine (BDZ), which is widespread but unsafe.
2. It is a study with the aims to stand as a guide for finding the right BDZ to give to a patient with dementia, and also the different pharmacokinetics of these drugs.
3. The study expresses the possibility of treating a patient with dementia with the right BDZ, along with adjuvant therapies.
INTRODUCTION
In the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), Alzheimer’s disease (AD [named after the German psychiatrist and neuropathologist, Alois Alzheimer, who first described it]), is considered as a major or mild neuro-cognitive disorder. It is estimated that about 50–70% of total dementia cases are due to this condition, while 10–20% are due to vascular dementia, and it is the fourth leading cause of death in industrialised countries.1
The lesions caused by AD can mainly be considered as two types: neurofibrillary tangles, consisting of paired helical filaments (pairs of tubulin associated unit), and senile or neuritic plaques, consisting of a protein called β-amyloid. In dementia, in addition to cognitive impairment there are, from the earliest stages of the disease, associated behavioural and psychological symptoms, which are grouped together as behavioural and psychological symptoms of dementia (BPSD).2 These can be anxiety disorders such as generalised anxiety disorder, psychomotor agitation, social phobia, and wandering, and they are often very severe and difficult to manage. Usually, BPSDs are not isolated but tend to appear in certain clusters. Clusters can be classified, according to the main symptoms of dementia, into predominantly affective, psychotic, hyperactive, and apathetic.
Several drugs have been found, and are in common use today, for the treatment of cognitive decline; however, the same cannot be said regarding the search for drugs for the management of behavioural and psychological disorders for patients with dementia. In fact, to date, there are no drugs that meet the efficacy and security pair for the treatment of BPSD. Drugs for the treatment of BPSDs, therefore, remains an unmet medical need.
To date, benzodiazepines (BDZ), anti-psychotics, mood stabilisers, and anti-depressants continue to be used in the clinic, despite their side effects.3 BDZs are among the most widely used psychotropic drugs; however, their use in the treatment of BPSDs appears to be an off-label regimen, as this treatment for BPSDs is not considered an authorised therapeutic, as indicated in their datasheet. The administration of BDZs, particularly in an elderly patient with dementia, can accelerate cognitive impairment, as the authors detail in this article.
This work is innovative as it focuses attention to the behavioural symptomatology that is associated with dementia, which is just as important to the symptomatology of cognitive impairment, which is studied more often. The review lends itself as a possible guide to follow for the treatment of BPSDs with BDZs and for choosing the right BDZ in relation to different pharmacokinetic parameters, in combination or not with adjuvant therapy.
MATERIALS AND METHODS
The present experimental thesis work consists of a systematic literature review by consulting the two medical-scientific databases: PubMed and Cochrane. The material is between the date of the first available article (1965) and the end of the search (August 2020). The search strategy was to use the following keywords: “benzodiazepines and dementia” and “benzodiazepines and Alzheimer’s disease.”
Inclusion Criteria
All available clinical trials concerning BDZs used for the treatment of BPSD and their possible involvement in increasing cognitive impairment were considered. No filter was applied regarding the date and duration of the study, or its possible follow-up. The focus was on clinical trials where the role of BDZs was observed specifically in Alzheimer’s dementia. The papers were selected based on tests for assessing the stage of dementia such as Mini-Mental State Examination (MMSE), as well as the Global Deterioration Scale (GDS), National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) Criteria, and International Classification of Diseases ninth revision (ICD9), etc. The last selection was made considering studies in which the age of patients was over 65 years.
Exclusion Criteria
The following were not considered for this review article: narrative and systematic reviews; meta-analyses; conference communications; book chapters; studies on forms of dementia other than Alzheimer’s dementia; studies in which the assessment of patients’ dementia stage was not done in relation to a particular rating scale or diagnostic criteria, as mentioned above; studies including patients with early-onset dementia (under 65-years-old). Duplicates were eliminated, as were papers that were not open access in the above databases and also papers that were written in a language other than Italian and English.
RESULTS
The search of the study material yielded a total of 792 scientific articles, including 183 from Cochrane and 609 from PubMed. First, all the duplicate articles were eliminated. Then, the authors proceeded to consider the remaining articles (693) according to the inclusion criteria. The authors only included clinical trials, such that narrative and systematic reviews, meta-analyses, conference communications, and book chapters were excluded. The authors considered clinical trials that were only concerned with AD; however, they did not consider other forms of dementia. The number was thus reduced to 89 clinical trials. These were then analysed in relation to the remaining inclusion criteria (articles in which patients are classified according to MMSE or other diagnostic criterion such as GDS or NINCDS-ADRDA and also articles where the patients were over 65 years old). Therefore, a total of 28 articles were included. Further analysis of the remaining clinical trials was completed according to the exclusion criteria, where unavailable articles or those written in a language other than English were not considered; thus, the authors found three clinical trials for qualitative synthesis (Table 1). Cognitive impairment was assessed by means of several rating scales (Table 2).
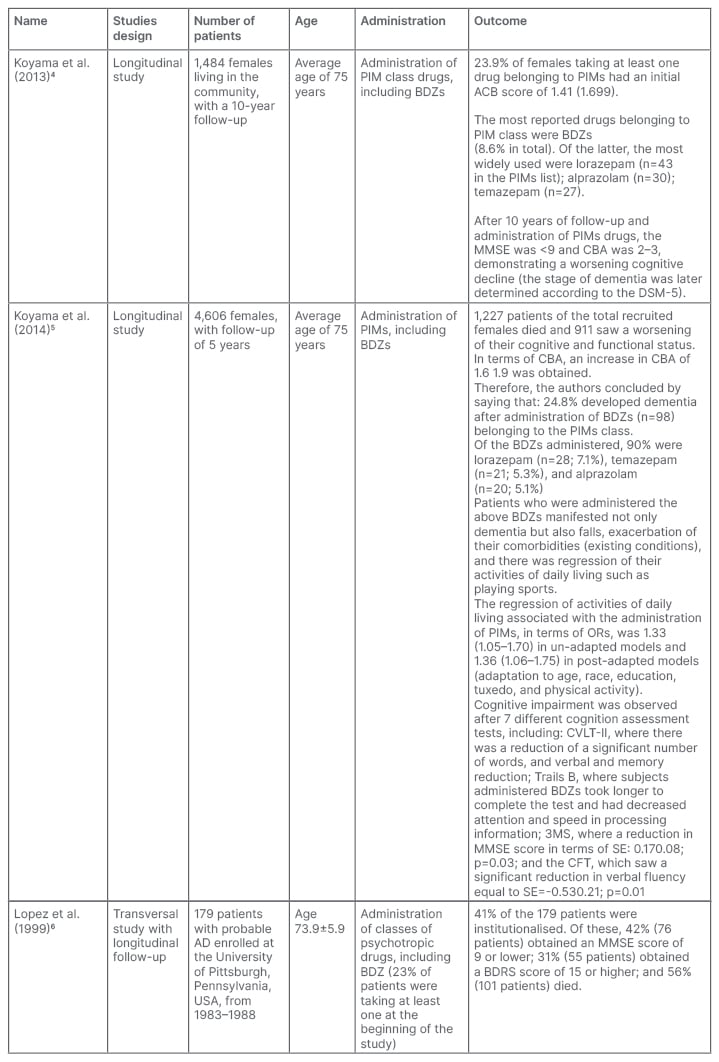
Table 1: Key features of the studies included in the qualitative synthesis.
ACB: Anticholinergic Cognitive Burden; AD: Alzheimer’s disease; BDRS: Blessed Dementia Rating Scale; BDZ: benzodiazepine; CFT: category fluency test; CVLT-II: California Verbal Learning Test, second edition; DSM-5: Diagnostic and Statistical Manual of Mental Disorders, fifth edition; IADL: impairment activity of daily living; MMSE: Mini-Mental State Examination; OR: odds ratio; PIM: probable inappropriate medication; SE: side effect.
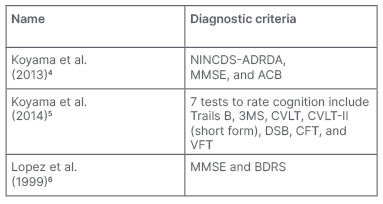
Table 2: Techniques used in the studies of these systematic reviews.
ACB: Anticholinergic Cognitive Burden; BDRS: Blessed Dementia Rating Scale; CFT: Category Fluency Test; CVLT: California Verbal Learning Test; CVLT-II: California Verbal Learning Test, second edition; DSB: Digit Span Backwards; MMSE: Mini-Mental State Examination; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; VFT: Verbal Fluency Test; 3MS: Modified Mini-Mental State Examination.
DISCUSSION
It is estimated that BDZs belong to a class of medications called probable inappropriate medications (PIM), which are inappropriate in the elderly.4,5
According to data from the Italian Medicines Agency (AIFA),7 50 daily doses of BDZ are prescribed per 1,000 patients, a trend that is increasing; it is approximately 8% higher than the previous year. In daily clinical practice for the pharmacological treatment of BPSDs, ‘short-acting BDZs’ (i.e., BDZs with a shorter half-life such as Lorazepam), are mostly used. In some cases, however, the authors also refer to the use of ‘long-acting BDZs’ and their major representatives such as diazepam. The former is mainly used for the treatment of insomnia, while the latter for the treatment of anxiety.8,9
For this systematic review, the authors have focused on 3 studies, two of which are longitudinal studies by Koyama et al.4,5 and the other is a cross-sectional study by Lopez et al.,6,10 with longitudinal follow-up.
The aforementioned drugs cause a significant increase in cognitive decline, according to a study by Koyama et al.,4 in addition to a worsening of other comorbidities in different ways that were associated with age-related changes in pharmacodynamics and pharmacokinetics. This cognitive decline and worsening of other comorbidities is also due to the use/abuse of multiple classes of inappropriate psychotropic drugs, resulting from the consequent problems arising from their interaction,11 despite the fact that guidelines are already available regarding polyadministration.12
According to Koyama et al.,4 as inappropriate drugs for older patients, BDZs might not only result in worsening cognitive decline of patients with AD; Koyama et al.4 indicate that the drugs may be a cause of it, or even result in both. To assess the role that BDZs play, worsening cognitive decline and the reached stage of dementia were observed through the MMSE in the former, and the latter was correlated with the Anticholinergic Cognitive Burden (ACB) scale. In fact, an increase in GABAergic tone, as determined by BDZs, may result in a decrease in cholinergic tone.13
Regarding the first rating scale, an MMSE score of less than 9 was obtained; while in regard to the ACB, the score was 2–3. These scores confirm the close association between the use of these PIMs and the net worsening of cognitive function of the patients under consideration, plus the worsening of psychological comorbidities at a high stage of dementia, which was later confirmed by a diagnosis made by a team of experts in accordance with the Diagnostic and Statistical Manual of Mental Disorders (DSMMD), created by American Psychiatric Association in 1994. In confirming the PIMs–BDZ association and increased cognitive decline, however, the authors ultimately failed to understand whether BDZs, in addition to leading to worsening cognitive status, may also be the cause, on par with previous studies.14,15
The 2014 study by Koyama et al.5 is a ‘continuation’ of the 2013 study, confirming that BDZs result in a high rate of adverse effects, both short-term16,17 and long-term.15 The focus is no longer on the dual risk of worsening cognitive impairment and mortality in older patients, but rather on the dual risk of worsening cognitive impairment and regression of activities of daily living (impairment of activities of daily living),18 associated with an increased anticholinergic load.
The worsening cognition of the patients considered was determined by assessment of the MMSE and correlated with the CBA; however, other cognitive status assessment tests were also used such as Trails B, Modified Mini-Mental State (3MS), California Verbal Learning Test (CVLT) second edition CVLT short form (CVLT-II), Digit Span Backwards (DSB), Category Fluency Test (CFT), and Verbal Fluency Test (VFT). These demonstrated worsening in terms of reduced attention and readiness in response to a test (Trials B); reduced verbal fluency and semantic memory (CFT and VFT); and reduced visual-spatial ability and orientation (3MS).
Regarding anticholinergic load (ACB), other recent studies have shown that worsening cognitive function in patient with AD may be associated with reduced cholinergic tone.15,19 Cholinergic neurotransmission is critical for cognition as the ‘pyramidal’ neurons of the septus or basal nucleus of Meynert communicate with cortical areas and the hippocampus by means of terminals that release acetylcholine. Some drugs used in the clinic for slowing cognitive decline are indirect cholinomimetics such as donepezil, rivastigmine, and galantamine.
Cognitive impairment is also increased by BDZ use through interference with neurodegeneration. It has been shown in a transgenic mouse model that chronic BDZ use can result in an increase in α42, a form of amyloid that is insoluble and thus capable of forming the senile plaques typical in AD.20 Despite this, the characteristics of the experimental studies still do not allow for definitive conclusions.
A study by Lopez et al.6 highlights the importance of treating BPSD for a patient with AD, as symptoms such as insomnia, psychosis, and agitation are predictive of functional decline, hospitalisation,10,21,22 cognitive decline,10,23,24 and even death. In the latter case, symptoms associated with an increased risk of death are depression;25 wandering and behavioural problems;26 and selected behavioural symptoms such as agitation, wandering, and hallucinations, which appear to play a predictive role in death.27
The findings of the study of Lopez and colleagues6 on the use of BDZs and their consecutive alteration of the normal course of AD pathology are similar to that of the study by Koyama et al.5 This is in terms of cognitive decline (cognitive impairment) due to reduced cholinergic tone and anticholinergic activity; and regression of activities of daily living, secondary parkinsonism, orthostatic hypotension, and sedation.
The dual risk of cognitive impairment and regression of activities of daily living is consolidated by identification of an advanced stage of dementia. An assessment is conducted by considering the MMSE score, which turns out to be very low indeed. For the assessment of reduced activities of daily living (such as the inability to play sports), the Blessed Dementia Rating Scale (BDRS), which turns out to be high, is considered instead. BPSDs are, therefore, important symptoms to be treated in a patient with AD due to the increased risk of cognitive impairment, functional impairment, and hospitalisation that these symptoms result in; however, BDZs are not so safe that they can be prescribed for dementia.
Some relatively recent studies8,9 have pointed out the increased risk of dementia after chronic exposure to BDZs, which is when a patient takes a BDZ for more than 3 consecutive months of drug therapy. In addition, the risk of increased cognitive impairment following chronic BDZ use may also be different according to the different BDZs considered (i.e., the risk of increased cognitive impairment varies according to the different pharmacokinetics of BDZ).8,9 In fact, several studies note that a meeting point between efficacy and safety of these drugs in the treatment of BPSD should be found in the use for short periods of time for short-acting BDZs such as lorazepam or oxazepam.28-30
Increased cognitive impairment after higher doses and prolonged times of drug therapy with long-acting BDZs such as diazepam has been demonstrated by several studies. Of particular relevance is the study conducted by Billioti de Gage et al.,9 in which a markedly different risk of acceleration of cognitive impairment is observed between the administration of a short-acting BDZ and a long-acting BDZ, as well as the difference between shorter drug therapy times and much more protracted times instead. In the present case, regarding the difference in pharmacokinetics, this pivotal study reports that the risk of increased cognitive impairment in patients with AD is 70% in those who are given long half-life BDZs, and the risk of increased cognitive impairment in patients with AD is 43% in those given short half-life BDZs. In terms of timing of drug therapy, however, it is reported that the risk of worsening and existing cognitive impairment is 50% in patients given BDZ for 3 months, and the aforementioned risk is even greater in patients treated with BDZ for 6 months (as high as 84%).
In the study by Sunderland et al.,31 a short half-life BDZ such as lorazepam at low doses and for a few weeks of treatment (2 weeks) did not result in accelerated cognitive decline.
Other more recent studies have shown that it is necessary to provide individualised treatment for patients with BPSDs.32 The latter could also include non-pharmacological therapies33 such as aromatherapy and music therapy,28,34,35 which, according to latest clinical data, present efficacy in managing agitation in dementia patients without the side effects of psychotropic drugs.
CONCLUSION
BDZs represent a widely used class of psychotropic drugs in the treatment of BPSD in patients with AD. They are very effective in treating these symptoms but, at the same time, they do not appear to be safe drugs. The clinical trials identified in this systematic literature review support that BDZs result in increased cognitive impairment in the AD patient. The possible reasons for this include a high rate of side effects such as amnesia, confusion, and sedation; increased GABAergic tone that, in turn, results in decreased cholinergic tone due to reduced acetylcholine release; possible increase in neurodegeneration due to increased synthesis of α42 (a form of amyloid predisposed to senile or neuritic plaque formation); an increased dual risk of worsening cognitive impairment and impairment in activities of daily living, patient mortality, and falls with potential hip fracture; and significantly increased risk of worsening cognitive decline due to the wrong choice of BDZ from a pharmacokinetic point of view, dosage (high), and timing of drug therapy (prolonged for 3 or even 6 months).
For the reasons stated above, BDZs are effective but unsafe drugs, which is why they are still administered for the treatment of BPSDs as an off-label regimen. Without resolving this for older patients with AD, this remains an unmet medical need. Subsequent studies are needed to determine the right choice of BDZ, a short acting BDZ, to be administered to an older patient with AD in the right doses and for an appropriate treatment period. In addition, individualised therapy should be preferred, including complementary therapeutic approaches (see aromatherapy and music therapy), which demonstrate efficacy in the absence of side effects.







