While vaccine development has lagged over the past decade, the pandemic revived this area of healthcare. What has been gained from COVID-19 that will inject new life into the vaccine space?
Words by Isabel O’Brien
A few short years ago, the international vaccine market was facing a demise that would irk even the most ambivalent starlet in Hollywood. Industry insiders were observing a decline in interest from investors, and the development and commercialisation of vaccines had reduced to only a handful of companies.
At the time, the pharmaceutical sector was accused of being too risk-averse and profit-orientated. On the flip side, governments were charged with relying on reactive rather than proactive policy making. The precise cause is murky, and may lie somewhere in between these two perceived offences, but as COVID-19 vaccines continue to restore normality and save lives around the world, could the space be primed for an epic revival?
The potential
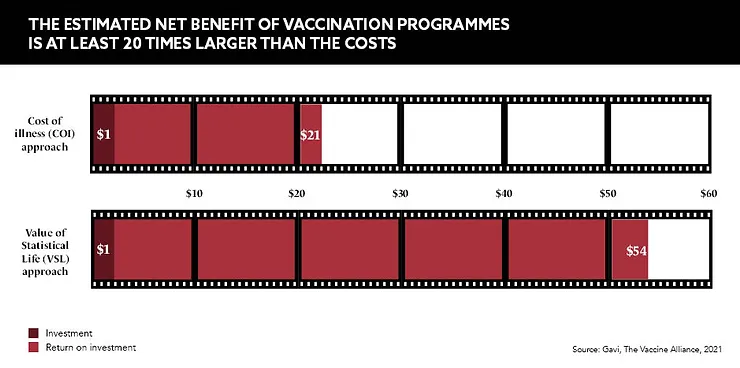
Over the past 10 years, Gavi, The Vaccine Alliance has been investigating the return on investment (ROI) for vaccines that could prevent 10 infectious diseases in 94 low- and middle-income countries. When considering the treatment costs, lost wages and productivity shortfalls that are incurred when a person is sick with a preventable condition, it found that the costs averted via immunisation programmes could amount to $828.5bn over the next decade.
COVID-19 has shown us what is actually possible in terms of how fast we can develop vaccines
“There are many other diseases where vaccines could be an extremely powerful preventive medicine,” says Lovisa Afzelius, Origination Partner, Flagship Pioneering, at the FT Live’s Global Pharmaceutical and Biotechnology Conference. The value of increased vaccination is unquestionable and, despite all its tribulations, the pandemic has sparked an opportunity for improved vaccine development across all infectious diseases. “Given the public health benefit and the health economics argument of vaccines, I hope that this momentum stays for a while,” says Nell Beattie, Chief Business Officer, VBI Vaccines, also speaking at FT Live’s conference.
Efficacy, speed and manufacturing
Before the global crisis, the vaccine to treat mumps proudly held the award for the quickest approval. Its conception took just four years, which is striking when compared to the average of a decade, but is dwarfed by the nine months it took for Pfizer and BioNTech’s COVID-19 vaccine to be anointed. The highest efficacy for a flu vaccine was also substantially lower than those for the COVID vaccines, pinpointed at 45% by Gavi.
“COVID-19 has shown us what is actually possible in terms of how fast we can develop vaccines, but also the efficacy levels that we can aim for,” says Rasmus Bech Hansen, Co-founder and CEO, Airfinity, also at FT Live’s conference. While vaccines were once tarnished by underwhelming efficacies and long development cycles, the pandemic has shown what is possible through the use of innovative development and approval processes. If crisis measures can be translated into routine procedures, the red carpet of preventative solutions could become swarmed with new and transformative vaccinations.
There are many other diseases where vaccines could be an extremely powerful preventive medicine
Manufacturing plants that were built and scaled to produce COVID-19 vaccines could also act as bases for future vaccine development. While governments have suffered criticism for their lack of foresight, they now have an opportunity to use these emergency facilities to produce all kinds of vaccines, harnessing the expanse of the infrastructure to ensure there is equitable access to new innovations. “A country could invest in and pay an insurance premium for an ongoing vaccine production base, and while we are waiting for the next pandemic, it could be producing vaccines that are needed but are not profitable today,” says Bech Hansen.
This would not only allow the world to be more prepared for future outbreaks, but it would circumvent “a scenario where you have all of this extra and unused capacity and resources lying around in a post-pandemic world”, says Beattie. She emphasises that as the pandemic highlighted infrastructure, manufacturing and access weak spots, it also identified the need to invest in innovation for the next generation of vaccines.
Public attitudes
The emerging scene is awash with positivity, but the industry also needs the public to call cut on vaccine hesitancy if new innovations are to be successful. Historically, hesitancy has blighted prophylactic vaccines in particular, as some individuals are reluctant to partake in global vaccination drives out of fear of side effects, or on ethical grounds pertaining to free choice.
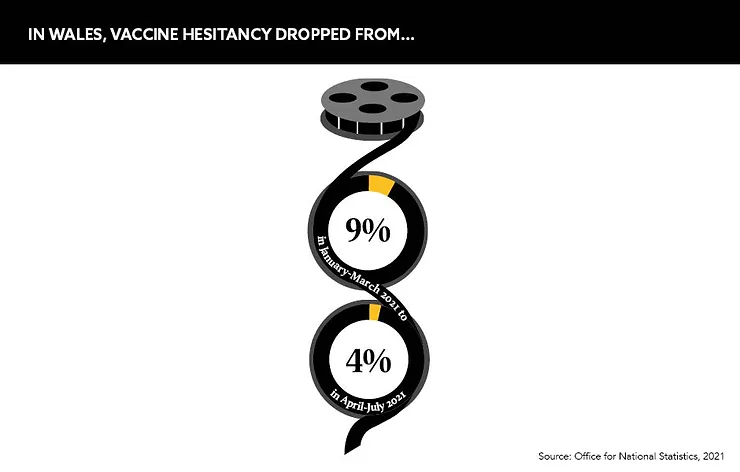
The issue has been well-documented during the COVID-19 crisis, but in reality, hesitancy is decreasing. For example, the UK’s Office for National Statistics reported that in Wales, reluctance from the public dropped sharply from 9% in January to March 2021 to 4% in April to July 2021, and other home nations saw similar downward trends.
“Hopefully, with the innovation in the vaccine space, we’ll see an uptick in the protection against all of these other vaccine preventable diseases, but also that you’ll start to see the general public take more ownership of their vaccination status,” says Beattie. While it is impossible to eradicate the sceptics completely, the COVID-19 vaccine effort has led to an increase of trust in these solutions, which could bode well for the innovations that are currently on the cusp of creation.
Looking to the future
As the industry settles into a new year, the impact of the pandemic and the revival effort by vaccine developers will not go unnoticed by the investors who contributed to the earlier regression. GSK hopes to deliver an HIV vaccine by 2030, and a new trial has been announced by the University of Oxford to investigate a more effective vaccine solution to Ebola. While suspected inertia from both the public and private sectors was stifling this area of healthcare previously, the eradication of red tape, increased manufacturing capabilities and a boost in public trust, is set to swing the spotlight back onto vaccines and their life-saving potential.
“The whole risk calculation has changed and that will lead to a continuing flow of investment, especially from governments around the world,” says Bech Hansen. The humble vaccine was crying out for a return to stardom, and the global crisis has created an opportunity for a seismic comeback.