Tackling health inequities by copying a leading COVID-19 vaccine is a complex, lengthy but potentially promising option to the current and future pandemics. But what are the roadblocks and how can replicators overcome these?
Words by Danny Buckland
The launch of a small-sized production facility might normally only cause ripples of interest and mild celebrations at the ribbon-cutting ceremony, but a new unit has thrown down monumental challenges for the pharmaceutical industry across its entire scientific, legal and ethical landscape. It is here, on an unremarkable business estate in Cape Town, South Africa, that a vaccine revolution for emerging nations has begun.
Afrigen Biologics, a consortium backed by the World Health Organization, is deploying a successfully reverse-engineered Moderna COVID-19 vaccine to create a route to locally produced vaccines for low- and middle-income countries (LMICs), which have had to rely on the generosity of foreign governments and big pharma to date.
The initiative aims to produce the first African-owned vaccine at Afrigen’s mRNA hub, with a 70 million doses a year target, and radiate the benefits out to 15 spokes across the continent, before expanding to areas of South America and eastern Europe with poor access to COVID-19 vaccines.
Dealing with disparities
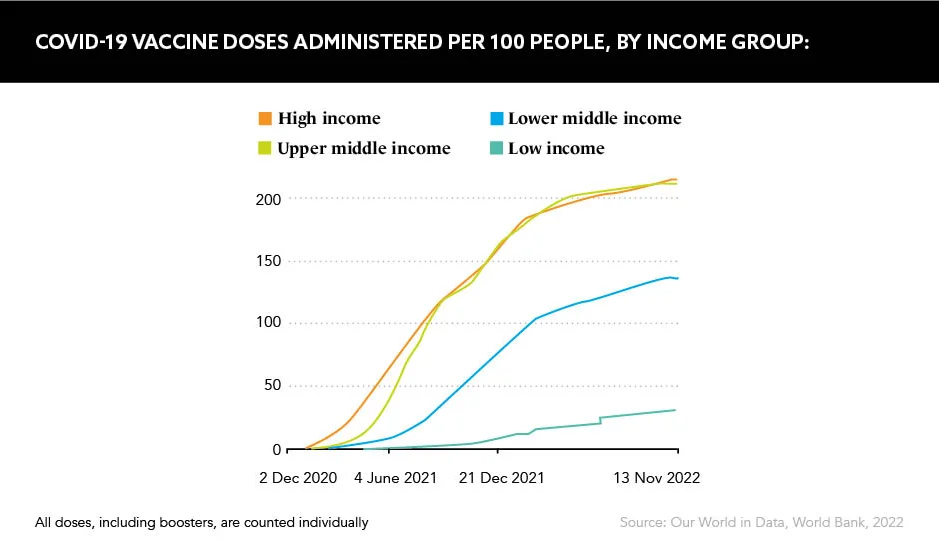
Huge disparities in vaccination rates around the world – 62% of the population had been vaccinated as of August 2022, but only 17% of people in low-income countries, according to Gavi, the Vaccine Alliance – are the propelling force behind this replicated vaccine, but concerns over bio-security and efficacy, plus intellectual property lawsuits, swirl around its infancy.
Afrigen could not strike a technology transfer agreement with Moderna which, although agreeing not to enforce its patent in 92 LMICs, wants to protect its vaccine. This original vaccine, along with Pfizer’s version, are predicted to generate at least $51bn revenue this year.
There have always been plenty of nay-sayers who don’t want the status quo to be disrupted
“The purpose is to build capacity to make mRNA vaccines in response to the pandemic and as part of a pandemic preparedness programme, which is a socio-economic imperative,” says Professor Petro Terblanche, Managing Director, Afrigen Biologics. “We are challenging the paradigm that you need large facilities to do something like this, but we are doing it without compromising quality. We had hoped this would be a tech transfer with turnkey technology but that didn’t happen, so it became a greenfields programme, growing everything from the ground up, which takes time.”
A positive dialogue
Prof. Terblanche accepts Moderna’s decision to decline the technology transfer as “it’s prerogative” and highlights that she will continue dialogue across industry to demonstrate the Afrigen vaccine is no commercial threat and could, in time, help generate more business for pharma companies.
The replicated vaccine has achieved good data throughout development and is ready to move onto clinical trials after the facility goes live on 15 December 2022. This will mark the start of a long-term ambition to establish a pipeline of mRNA vaccines that will bring better equality to Africa.
“We are working on a pipeline of products that can be put through this platform for commercial benefit because that is where sustainability will come, while we need to design facilities that you can keep in pandemic preparedness for when, not if, we need them,” Prof. Terblanche explains. “We are very excited by the potential and determined to realise it.”
Charles Gore, Executive Director, Medicines Patent Pool – the United Nations organisation created to increase the development of and access to life-saving medicines in LMICs – comments: “It is a project with a big ambition and so, yes, there are daily problems, challenges and roadblocks – some bigger than others. Some are scientific, others are logistical and then there are those that are political.”
It is a project with a big ambition
He adds that a range of governments have provided financial support for the project and that Afrigen has developed a strong network of supportive organisations, which is key to this kind of strategy.
“Throughout history when new ways of doing or thinking have come about, there have always been plenty of nay-sayers who don’t want the status quo to be disrupted,” Gore notes. “With this project we are doing things differently. We are empowering LMICs to develop, make and share their own vaccines. This disrupts the status quo, and we will have those who will believe it can’t be done. Our job is to show that it can and will be done in partnership and with the excellent scientists in South Africa and in LMICs.”
Transformative potential
The vaccine replication exercise was uncharitably characterised earlier in 2022 as “like copying a Louis Vuitton handbag”, as reported in the international press, but Moderna is as committed to helping LMICs as it is to protecting its hard-earned and long-researched vaccine IP.
The US-based company has a mission “to create a new generation of transformative medicines for patients” and states that 25% of the 800 million COVID-19 vaccine doses have reached LMICs.
In October 2020, Moderna became the first and only pharma company to pledge not to enforce its COVID-19 vaccine patents or IP rights in 92 LMICs, and the company recently announced it was working on a refrigerator-stable version of its vaccine, which it says will also drastically reduce supply chain problems for these nations.
“We will internally fund and build an mRNA manufacturing plant in Kenya capable of producing up to 500 million doses per year so that Africa has its own local source of vaccines, and we can start to build an ecosystem of individuals with the knowledge of how to manufacture this new generation of medicines,” a spokesperson says.
These are early days in establishing a framework for equitable global healthcare and, although they are tinged with threats of disputes and lawsuits, and other societal factors are at play, the success of the Afrigen project will go a long way to carving better access to vaccines and transformative therapies across LMICs.