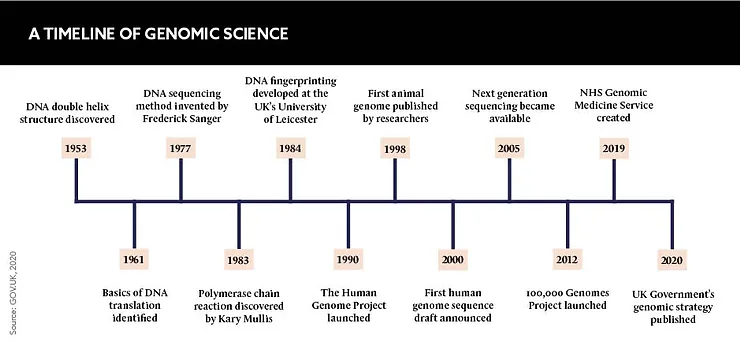
The potential for genomic medicine is vast, but is progress being hampered by a lack of trust from the general public? Pharma must work to combat the public’s ethical, data and equity concerns to ensure genomic medicine reaches its life-saving potential
Words by Isabel O’Brien
What do politicians, relationships and genomics all have in common? It may sound like the opening to a well-rehearsed joke, but these entities share a pivotal foundation: trust. Each of these institutions are likely to topple without a strong basis of trust, but while political figures and romantic partners are ultimately replaceable, the stakes are far higher in the world of genomic medicine.
The use of genomics in healthcare is unrivalled by any other treatment type currently available, yet a lack of public trust is threatening to derail its life-saving potential. “With genomic medicine, we are advancing science with the potential for one-time treatments that deliver lifelong benefits to patients with genetic diseases,” says Sandy Macrae, CEO, Sangamo Therapeutics. “It’s a thrilling possibility but, as with any advancement, some people fear the unknown.” How can the pharmaceutical industry tackle the trust gap in genomic screening and therapies?
Seeds of doubt
Over the years, multiple studies have been conducted on public attitudes to genomic medicine, and often conclusions have been negative. Two reports, conducted in 2017 and 2022 respectively, found that people with religious views are more likely to object, likening it to ‘playing God’. Other research has revealed that the public fear that their genetic data may be used against them by medical insurance companies, or that genomic screening and therapies will exacerbate health inequities by only being made available to a privileged few.
“Historically, there have been examples where the broad concept of genetics has been used as an excuse to promulgate discrimination,” says Narimon Honarpour, Vice President, Global Development, General Medicine, Amgen. “The mistrust today partly comes from that history, as well as concerns on how such information could be used to disadvantage individuals or groups.” A trust gap exists, but a knowledge vacuum is largely to blame. This is no clearer than in public confusion between genomic medicine and genomic enhancement. Many hear ‘genomics’ and instantly think ‘designer babies’.
Tackling misconceptions
In genomic medicine, misconceptions can be harmful and prolific, and myth-busting is crucial. Conversations need to be directed away from early-life screening and towards potential alternative avenues such as those in oncology, infectious diseases and other forms of screening.
For example, a real-life trial took place at St Mary’s Hospital, Manchester in 2020 that screened newborns admitted to intensive care for a rare gene that could cause them to go permanently deaf if a routine post-birth emergency antibiotic was administered. Despite having limited coverage, the pilot scheme was hugely successful in achieving its objectives and proved to be both accessible and affordable – a stark contrast to common public perceptions of genomic medicine. As Saptarsi Haldar, Vice President, Cardiometabolic Disorders, Amgen, says: “We need to do a better job of this; when we have successes, we absolutely need to highlight them to the public.”
It’s a thrilling possibility but, as with any advancement, some people fear the unknown
When therapies such as Novartis’s one-time treatment for spinal muscular atrophy hit the market with record breaking price tags – Zolgensma is priced at $2.48 million a dose and is now the most expensive drug in the world – it can understandably lead to public outcry around profit margins and health equity. However, there is plenty of scope for the industry to educate people on the time and expertise required to develop these therapies, and how the system needs to adapt to ensure these highly advanced treatments can be accessed by all.
“Our current system is built for transient treatment of acute conditions and sustained, long-term treatment of chronic ones,” explains Macrae. “Therefore, it will be necessary for the system to change to better accommodate genomic medicines, which come with upfront costs for one-time treatments that can potentially deliver lifetime benefits.”
A data drought
Ethics and pricing aside, the industry needs people to wilfully hand over their genetic data to sustain research efforts and recruit for gene-based clinical trials. Yet, many individuals who would gladly share other forms of data are wary about giving up their genetic information.
“Providing more awareness on how this information is obtained, analysed, used and stored could be quite helpful,” says Honarpour. “With that, the medical and scientific community would have a greater opportunity to understand how genetics affect risk of disease and how the risk may be different for different people.” He adds that as the pharma industry is one of the most regulated in the world, it has some of the best mechanisms to keep sensitive information safe.
When we have successes, we absolutely need to highlight them to the public
“The biopharma sector can also educate the public on how de-identified genetic information can tangibly enable drug discovery, development and personalised medicine,” adds Haldar. De-identified genetic information can be just as valuable to genomic researchers, and the public needs to be informed that they could propel the future of genomic medicine without relinquishing an ounce of personal privacy.
Limitless potential
Genomic medicine could one day cure currently incurable diseases, relieving patients with conditions such as sickle cell disease from a lifetime of pain and hospital stays. On a smaller scale, it could enable doctors to prescribe hayfever medication tailored to an individual’s genome, or help women to select the perfect hormonal contraception for their genetics.
“Trust in science is needed,” concludes Macrae. “Only with trust in genomic medicine can we run clinical trials, build the necessary infrastructure and secure needed investments to ultimately bring new treatments to broad patient populations.” The potential is enormous, but the industry must construct a sturdy foundation of trust before the power of genomic medicine can be fully realised.