Getting compliance right in the US pharmaceutical landscape is a lot like cracking a code. Find out the right combination of strategies to protect and amplify your medical content while regulators watch on
Words by Jade Williams
For pharmaceutical companies, promoting new medicines in the US involves a delicate balance between innovation and compliance. With stringent regulations and a broad array of oversight bodies, ensuring that research is shared without legal risk can require a keen eye—and a careful hand.
Navigating this landscape requires strategic planning to maintain corporate credibility while adhering to regulatory guidelines. What key considerations should pharma companies keep in mind before releasing new research in the US market, and how can they stay ahead of potential compliance pitfalls?
The broad scope of regulatory oversight
While the FDA’s Office of Prescription Drug Promotion (OPDP) is a key player, multiple regulatory bodies are involved in monitoring how product research is presented by pharma. These include the Federal Trade Commission (FTC), the Department of Justice (DOJ), the Department of Health and Human Services (HHS) and others, which may monitor whether companies are publishing content that promotes off-label indications or publishing misleading content.
“The HHS and the DOJ often focus on the overall intent and promotion rather than just the content of the publication,” notes a US compliance expert. “These agencies may ask for a review of everything from a company’s publication strategy to KOL usage to how sales representatives distribute materials.”
Regulators focus on how publications and other materials are integrated into a broader promotional strategy, especially if it leads to off-label reimbursement issues. They are also less focused on the act of publishing itself, and more on how these publications are used or interpreted by HCPs. Even hinting at an off-label use or misrepresenting clinical data can result in regulatory scrutiny.

Choose an expert partner
Medical journals are the cornerstone of research dissemination, alongside congresses and other medical meetings. When selecting a journal to partner with, it’s essential to choose one with a strong reputation and a solid grasp of compliance. The journal should be able to clearly explain how the content will be disseminated and targeted to the appropriate audience.
This is where AMJ, the US arm of EMJ, excels. As an open-access publication that promotes free medical education, they have developed multiple strategies to ensure content reach is both curated and controlled. With extensive experience working with various companies across the sector, the AMJ team is also well-equipped to assist with bespoke requests as they arise.
“As an open-access platform, we work closely with clients to ensure their content reaches the right audience while adhering to the highest regulatory standards,” says Alexander Skedd, Vice President, Customer Success, AMJ.
Satisfy the review team
However, internal compliance can be the real hurdle to overcome. Increasingly, gaining approval for a promotional or medical education project requires input from multidisciplinary players.
From regulatory to marketing, many departments may need to review project plans to ensure they meet not only FDA standards, but also the requirements of other regulatory agencies throughout the launch timeline. But when trying to get a project over the line, what questions can you expect from the relevant stakeholders?
Regulatory
- Is the publication aligned with FDA guidelines?
- Does the study design and data meet regulatory standards?
- Are there any regulatory red flags in the content used?
Legal
- Is the content in compliance with advertising laws?
- Does the distribution plan for reprints, and will these raise concerns around improper promotion?
Medical affairs
- Do the results reflect the study design?
- Are the conclusions scientifically sound and free of bias?
- Does the content remain focused on scientific exchange, rather than promotion?
Marketing
- How might the content be interpreted by HCPs?
- How does the publication tie into the product’s marketing position – could it be perceived as indirectly promoting off-label use?
- Are there any aspirational claims or language that have crept into the content?
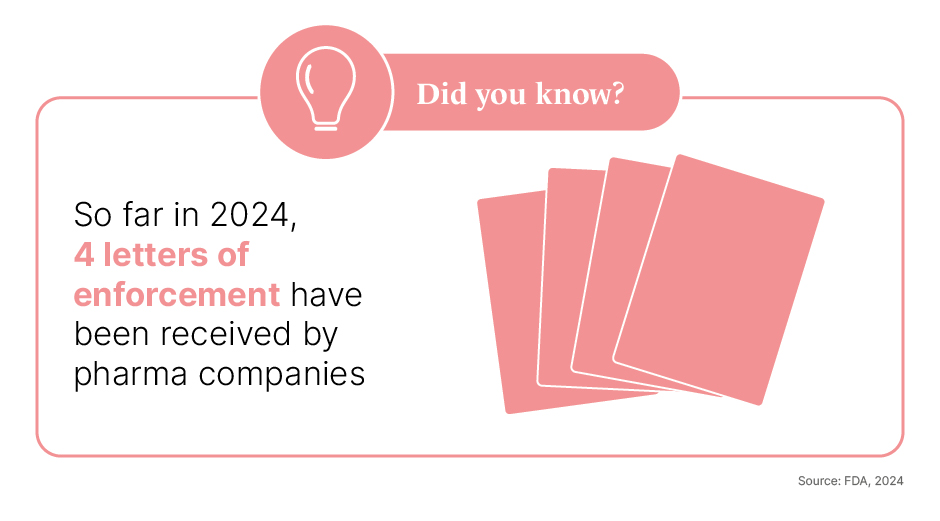
Avoid promoting off-label uses
Once approval has been secured and parameters set, it’s important to keep compliance in mind when selecting content for publication, particularly in relation to off-label promotion.
When publishing clinical trial results, the focus must remain strictly on the data, avoiding any implication of efficacy or safety beyond what the FDA has approved. “The FDA primarily looks for compliance red flags: are studies appropriately powered, and do they follow robust designs?” notes the US compliance expert. If a trial is designed for a specific condition, the findings must not be stretched to suggest broader applications.
Any language that could be interpreted as promoting or encouraging off-label use also needs to be carefully reviewed and removed. While it may be tempting to present data in the most favourable light for a new product, drawing conclusions that go beyond the study’s design risks attracting regulatory scrutiny. A peer-reviewed medical journal such as AMJ should flag and push back if such content is submitted.
Moulakshi Roychowdhury, Global Head of Regulatory Affairs, Advertising & Promotion, Allergan Aesthetics, AbbVie, underscores the importance of scientific rigor. “A publication is only useful to the medical community if it is presenting new and novel information that will ultimately benefit patient care.” While promoting all findings might seem desirable, “the scientific integrity and the goal to help patients is what should drive the publications and the conclusions therein.”
The compliance expert concludes: “HHS and DOJ penalties can be severe, with billions in fines if there is off-label promotion leading to improper reimbursements.” It is essential to catch compliance issues early to avoid enforcement risks. The review process must also account for how the article might be interpreted beyond its immediate scope.
Final word
Navigating the complexities of scientific discovery and regulatory compliance demands careful attention. While publishing in medical journals offers a powerful platform, it can also present significant challenges in the US compliance landscape.
Success depends not only on the quality of the data, but also on how accurately and transparently it is presented. As Roychowdhury advises, companies must prioritise “scientific integrity and the needs of patients”, and choosing the right partner to help with this mission will ensure that no conflicts of interest or biases can undermine a company’s work.
Just as regulatory codes are strict, pharma’s commitment must be unwavering—to transparency, precision, and intent—ensuring that compliance remains secure.









