Meeting Summary
This year’s 39th International Symposium on Diabetes and Nutrition in Anavyssos, Greece, hosted a series of presentations and plenary lectures with a focus on the effects of weight loss, micronutrients, nutritional supplements, and alternative dietary patterns in the prevention and management of Type 2 diabetes (T2D) and cardiovascular risk reduction. Michael Lean discussed how diabetes remission can be achieved through weight loss using a low-energy diet (LED) or very low-energy diet (VLED), accompanied by continued long-term support from specialised healthcare professionals. Jeffrey Mechanick discussed the importance and impact of early intervention on T2D and cardiovascular disease (CVD). He emphasised that T2D should be seen not just at the point of disease, but on a spectrum from prediabetes to complications, with early interventions having significant impact on not only the progression of T2D, but also into the latter stages.Simin Liu presented an integrative multilevel framework for causal inference to personalise cardiometabolic health, highlighting recent work investigating the roles of dietary minerals, environment metals, and genomics in relation to cardiovascular disease and diabetes.
Several of the presentations included discussion of specific interventions. Daniel West discussed the use of whey protein (WP) and how it can help control postprandial glycaemic excursions (PGE) in people with T2D controlled on oral antihyperglycaemic drugs. Following this, Andrea Hawkinson discussed how a new supplement, mulberry leaf (Morus alba) extract (MLE), can significantly lower postprandial glucose response, as well as early insulin response, highlighting the need for further studies to evaluate its efficacy in people with T2D. Philip Atherton showed studies providing evidence that protein and essential amino acid (EAA) supplementation can help support muscle mass, which is especially essential for older people with T2D and sarcopenia. Finally, Jose-María López-Pedrosa spoke about how a supplement containing slow digestible carbohydrates (SDC), arginine, lysine, and β-hydroxy-β-methylbutyric acid (HMB) can help preserve muscle mass, as well as improve insulin resistance, in a rat model of diabetes.
Weight Management
Michael Lean
Weight gain and obesity are major drivers of T2D.1-3
Sustained Weight Loss Can Lead to Type 2 Diabetes Remission
T2D remission (an HbA1c <48 mmol/mol without the use of glucose lowering medications for 6 months), improvements in cardiovascular risk factors, and decreased T2D-associated mortality can be achieved through sustained weight loss.4-7 As such, weight loss was a key recommendation of the 2010 Scottish Intercollegiate Guidelines Network (SIGN) guidance on the management of obesity, to which Lean contributed.7
Weight management is not only about weight loss, but also goal maintenance, prevention of weight gain, and minimising cardiometabolic risks.7 To achieve such goals, Lean emphasised the need for people with T2D and with overweight or obesity to be supported with evidence-based weight-loss treatments.
The Utility of Very Low-Energy Diets
Evidence from an umbrella meta-analysis review of randomised controlled trials for achieving weight loss in people with T2D showed that a VLED (400–500 kcal/day) and LED (1,000–1,500 kcal/day) are associated with greater weight loss than control (reduced energy) diets, while high- or low-carbohydrate, or high-protein diets, are not.8
In the DiRECT trial, led by Lean and Roy Taylor, 149 patients were given a total diet replacement intervention (825−853 kcal/day) for 3–5 months, followed by 2−8 weeks of structured food introduction, all provided and monitored by trained practitioners. Antidiabetic and antihypertensive medications were discontinued at study initiation, but reintroduced if needed. Remission of diabetes was achieved by 46% of the intervention group versus 4% of a standard practice care control group (n=149; p<0.0001).4
Sustained Support is Needed
Few studies evaluating dietary intervention and diabetes remission have been carried out for ≥12 months and, among those, remission rates over time may not be sustained.8 For instance, a Mediterranean-type diet study showed HbA1c lowering and weight loss, and a T2D remission rate of approximately 15% at 1 year, but following this, reported that rates fell over time.9
While the DiRECT trial was successful, Lean highlighted that a VLED can be difficult to follow and sustain. The vital elements to success are the skill and empathy of a healthcare practitioner and long-term support by a team with a consistent message. As such, he called for diabetes and weight-loss management practitioners to work together.
Conclusions
Achieving and maintaining weight loss is key for people with T2D and with overweight or obesity, as it can lead to disease remission and reductions in cardiovascular and mortality risks.
A VLED, supported by trained practitioners, can help with weight loss, but sustaining weight management requires ongoing support.
Dietary Patterns and the Role of Nutritional Supplements or Products in Diabetes Care: An Overview
Jeffrey Mechanick
In a chronic disease model, genetic and environmental risk factors, along with social determinants of health and transcultural factors, can lead to a predisease stage, which, if unchecked, can manifest and lead to complications.10
Type 2 Diabetes Needs to Be Viewed as a Disease Spectrum Over Time
With this disease model in mind, insulin resistance, prediabetes, T2D, and associated cardiovascular/metabolic complications can be envisaged as a spectrum over time instead of in isolation.11 As such, T2D interventions should occur in the early prevention stage as opposed to the active disease or complications stage, migrating preventive care from tertiary to secondary to primary settings, or even before.
Type 2 Diabetes Care Should Include a Structured Lifestyle Medicine Approach
According to Mechanick, a comprehensive care plan for a person with T2D must include a structured lifestyle medicine approach, with optimised nutrition and healthy eating patterns as a cornerstone. Dietary deficiencies with respect to micro- and macronutrients have been identified in people with T2D, and a review of diabetes-specific nutrition formulas provides clinical evidence for justification of their use.12
Nutrition Needs to Be Analysed as a Whole to Establish a Healthy Eating Pattern
Using a computer database, molecular components of food can be discovered.13 However, Mechanick stated that nutrients should not be considered individually, but rather as a network analysis of a person’s food metabolome that “can help identify the points that are opportunities for compounds to intervene to effect that network.” By doing this, a healthy eating pattern can be suggested that will contain all the nutrients required.
In an American Association of Clinical Endocrinologists (AACE) guideline, Mechanick and colleagues laid out recommendations for the clinical use of dietary supplements and nutraceuticals.14 These guidelines have been translated into recommendations in guidelines for T2D management and risk stratification.15,16
Conclusions
T2D should be envisaged as a spectrum from predisease to complications, so as to understand it in terms of a driver-based, chronic disease model.
By analysing and understanding a person’s individual food metabolome, individualised recommendations can be given to help at any stage along the T2D spectrum.
Can Frequent Whey Protein Supplementation Improve Glycaemic Outcomes in Type 2 Diabetes?
Daniel West
T2D is associated with elevated fasting plasma glucose (FPG) levels and increased PGE.17 Controlling this, according to West, is vital, as data shows that postprandial glucose response is not only a predictor of HbA1clevels18 and contributor to glycaemic variability,19 but also a predictor of future cardiovascular events20 and economic burden.21
Whey Protein as a Means to Reduce Postprandial Glycaemic Excursions
One product being investigated with regard to its actions on reducing PGE is WP.22 Recent studies have shown that WP slows gastric emptying, which in turn reduces the rate of digestion of glucose into the system23,24 and hepatic glucose output.24,25 WP has also been found to have a stimulating effect on insulin secretion, via stimulation of pancreatic β-cells,26 eliciting an increase in glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide.27,28 According to West, “all of these contribute to potentially improving postprandial glucose.”
Clinical Trials of Whey Protein
In a clinical trial with WP, 15 people with T2D, controlled either with a sulfonylurea or metformin, ingested 50 g of WP isolate or a control formulation 30 minutes before a high-glycaemic index breakfast. Results showed the WP formulation compared with the control significantly suppressed PGEs, as assessed by incremental area under the curve (iAUC) at 0−30, 60−120, and 0−180 minutes, (-22%, -29%, and -28%, respectively). Additionally, the insulin iAUC at 0−30, 60−120, and 0−180 minutes were higher with the WP formulation compared with the control (96%, 107%, and 105%, respectively) as well as the total/intact GLP-1 response iAUC (154%/240%, 137%/332%, and 141%/298%, respectively).27 This effect was less pronounced when WP was taken with a meal.28
In another study, the effect of WP on second meal effect was assessed in 11 males with T2D, controlled with lifestyle or oral antidiabetes medications. Participants consumed a supplemental drink of either 15 g of WP hydrolysate, intact WP, or placebo control, prior to consumption of a mixed-macronutrient breakfast and lunch. Compared with the control, the WP drink showed significant reductions in PGEs at both breakfast and lunch, but not at the evening meal when no drinks were consumed, suggesting there was not a second-meal effect. Notably, however, compared with the control, the WP hydrolysate drink was associated with a faster time to peak amino acid concentrations.28
Formulising a Whey Protein Supplement for Convenient Consumption
While the formulation used in this and the previous clinical study reduced PGEs, it was problematic due to the dose amount needed to be consumed, taste, and convenience. As such, West’s team worked to formulise whey hydrolysate into a palatable drink. To trial this formulation, 18 participants with T2D, predominately male and controlled either with lifestyle or oral antidiabetes medications, consumed either the test WP drink or control (no WP) matched in taste and consistency. The drinks were consumed three times a day, within 10 minutes of a meal, for 1 week.29 At the end of the intervention, the WP group compared with the control group showed approximately 10% less time spent hyperglycaemic (>10 mmol/L) and 2 hours per day more in euglycaemia. Additionally, supplement adherence was high (98%) and with no adverse events reported.29
West proposed that to determine long-term efficacy in patients with T2D on insulin or other oral antihyperglycaemic agents, longer-term studies are required with endpoints such as change in HbA1c, body mass, and impact on pancreatic β-cells.
Conclusions
Effective interventions targeting PGE in people with T2D are needed due to their association with improved glycaemic control and reducing cardiovascular risk and future cardiovascular events.
A Randomised, Placebo-Controlled Crossover Study to Evaluate Postprandial Metabolic Effects of Mulberry Leaf Extract, Vitamin D, Chromium, and Fibre in People with Type 2 Diabetes.
Andrea Hawkinson
Glycaemic management to reduce PGE in T2D is important, not just in terms of symptomatic management but also long-term cardiovascular risk reductions.30
Using Mulberry Leaf Extract to Control Postprandial Glycaemic Excursions
MLE, with origins in traditional Chinese medicine, contains sugar analogues (iminosugar alkaloids), with 1-deoxynojirimycin (1-DNJ) being the most abundant. 1-DNJ has been shown to slow glucose absorption by competitively blocking the active site of digestion and the polysaccharide-degrading enzymes, such as α-glucosidases.31 Other glucose-lowering mechanisms of MLE have also been described, associated to other functional ingredients including rutin, caffeic acid, chlorogenic acid, and quercetin.
Clinical Study of Mulberry Leaf Extract
Methods
The double-blind, placebo-controlled, crossover study led by Mafauzy Mohamed from the Universiti Sains Malaysia (USM), Kubang Kerian, Malaysia, aimed to test the incremental effect of MLE on the reduction of postprandial glucose and insulin levels in people with T2D of Asian origin (data on file; NCT04877366).32 There were two study sites (Singapore and California, USA) that enrolled adults (≥18 years) with T2D, either drug-naïve or metformin-treated (500−3,000 mg), with a HbA1c between 6.5% and 10.0%. Exclusion criteria included a BMI of ≥35 kg/m2, FPG >200 mg/dL, and/or estimated glomerular filtration rate <60 mL/min/1.73 m2 (data on file).32
At each study site, participants received either a 2 g powder containing 250 mg MLE, 12.5 mg DNJ, 1.75 g fibre, 0.75 mg vitamin D3, and 75 mg chromium or a placebo powder without MLE. The powder was sprinkled on top of a 350 kcal breakfast meal consisting of two slices of white bread, 20 g gouda cheese, and 250 mL apple juice (10.0 g protein, 6.5 g fat, and 55.4 g carbohydrates) and was consumed within 15 minutes. At the first study visit, all participants consumed either the test or placebo powder, with the alternative given at a second study visit (data on file).32
Blood glucose and insulin response were assessed over 3 hours (0, 15, 30, 45, 80, 120, and 180 minutes). The study’s primary endpoint was to assess the effects of the MLE powder on the iAUC (0−1, 0−2, and 0−3 hours) of PGE. Secondary endpoints included assessment of the effects on iAUC of the postprandial insulin response. Sample size was calculated to show a difference of 15% in the iAUC of PGE between the two arms. The iAUC was calculated using the trapezoid rule and comparison of treatment means, using the mixed model approach with product, site, and period as fixed effects, and participants as random effect (data on file).
Results
A total of 30 participants were randomised, with 29 completing both interventions. Baseline characteristics between the two groups were similar, with an average age of 58.9±11.3 years, a mean BMI of 26.5±4.4 kg/m2, and waist circumference of 92.9±11.2 cm. The overall mean FPG was 7.1±1.5 mmol/L, HbA1c was 54.1±5.1 mmol/mol, and systolic/diastolic blood pressure was 127±15/77±10 mmHg. There were some between-site differences, with the USA participants having a greater prevalence of obesity, and Singapore participants having slightly more dysglyceamia and lower estimated glomerular filtration rates (data on file).
Results (Figure 1) showed that the MLE blend, relative to placebo, significantly lowered the glucose iAUC curve by 19.5% at 1 hour (difference from control: -0.59 [95% confidence interval (CI): -0.83 and -0.34] mmol/L by hour; p<0.0001); 16.6% at 2 hours (difference from control: -1.21 [95% CI: -1.80 and -0.62] mmol/L by hour; p=0.0001); and 14.9% at 3 hours (difference from control: -1.43 [95% CI: -2.35 and -0.50] mmol/L by hour; p=0.0032). MLE also significantly reduced insulin response by 23.7% at 1 hour (difference from control: -3.41 [95% CI: -6.34 and -0.48] mlU/mL by hour; p=0.0236), but not at 2 hours and 3 hours, suggesting no overall effect on insulin response.
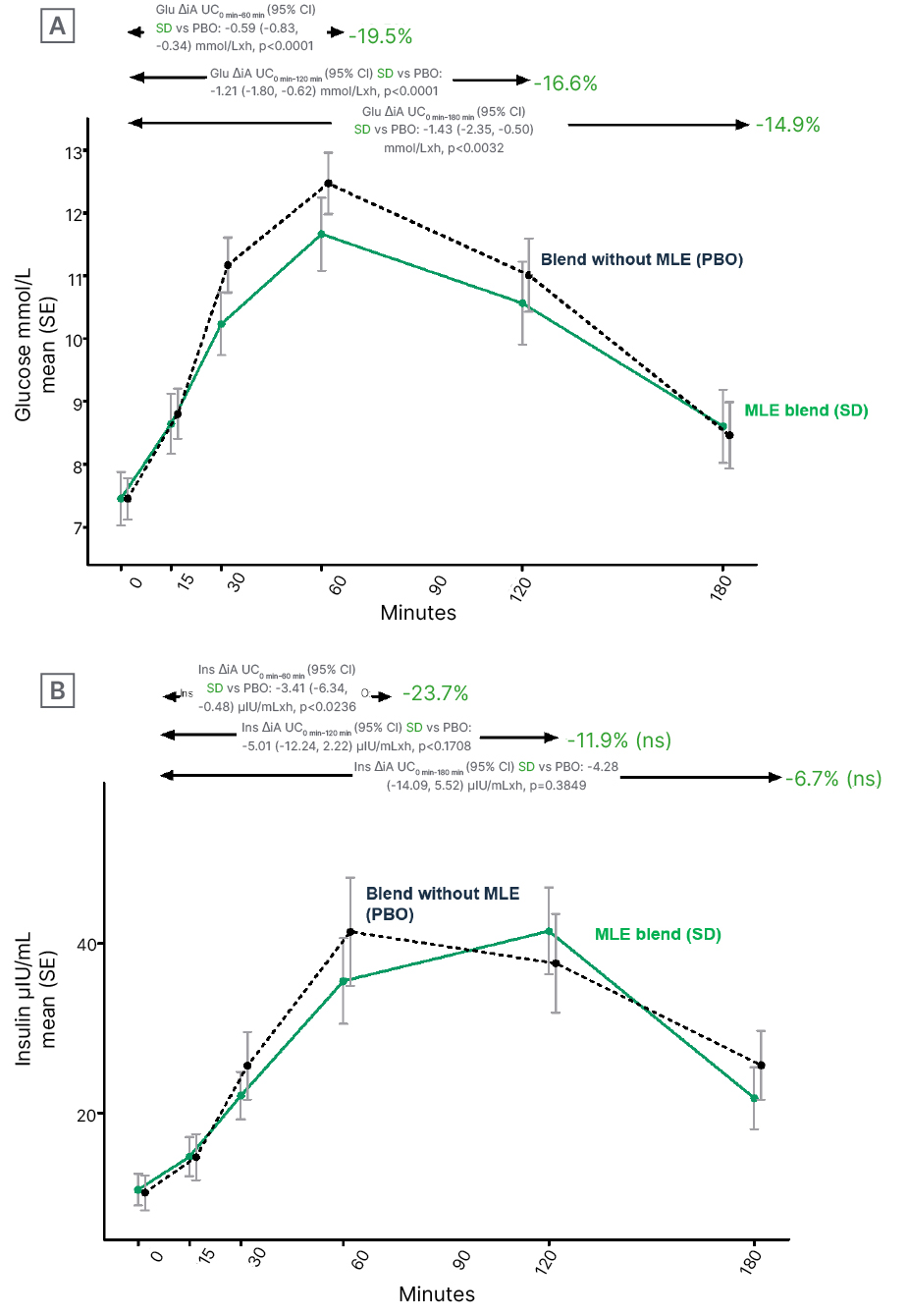
Figure 1: Effects of mulberry leaf extract on A) glucose and B) insulin trajectories.
AUC: area under the curve; Glu: glucose; Ins: insulin; MLE: mulberry leaf extract; ns: not significant; PBO: placebo; SD: standard deviation; SE: standard error of the mean; vs: versus.
One adverse event of fatigue, which lasted 4 days, was reported in the MLE group. Limitations of the study were that only a single dose was tested, the non-MLE ingredients in both formulations may have had an effect on glucose metabolism, and the study population only included adults of Asian origin (data on file).
Conclusions
Significant reductions on overall glycaemic burden and early insulin response following ingestion of the MLE-containing blend suggests the blend could be used as a dietary adjuvant to improve postprandial glucose response.
The findings support a reduced glucose gastrointestinal absorption effect of MLE, which could be mediated by DNJ. Longer-term studies are needed to understand the full translational metabolic impact of MLE.
An Integrative Multilevel Framework for Causal Inference to Personalise Nutrition for Cardiometabolic Health: Minerals and Metals?
Simin Liu
CVD-related deaths in the western world have fallen over the last 50 years due to advances in medicine and public health actions.33 However, there are still a number of known risk factors identified by the America Heart Association (AHA) as being poorly controlled, including smoking cessation, physical inactivity, blood pressure ≥120/≥80 mmHg, BMI ≥30, lack of a healthy diet, total cholesterol ≥200 mg/dL, and FPG ≥100 mg/dL.34
The Need for Diversity in Diet
The U.S. Department of Agriculture (USDA)MyPlate initiative recommends that dietary patterns should be diverse and include a variety of nutrient-rich fruits, vegetables, whole grains, and protein.35 These recommendations stem from the failing in findings from a study where only one component of the diet was controlled. A subgroup of females from the Women’s Health Initiative Study participated in a large, USA-based, randomised controlled trial investigating dietary modification in postmenopausal females (aged 50−79 years; n=48,835). It was found that when energy from fat was lowered to 20% from ≥35%, there were no differences in primary outcome measures of coronary heart disease and total mortality occurrence. A confounding factor in the intervention, Liu highlighted, was that fat energy was mostly replaced with carbohydrates (half of which were sugar), not proteins.36
An Integrated View of Cardiometabolic Health
According to Liu, “there is no hope of identifying a gene that puts you at any substantial risk of developing disease without the environmental context of nutritional behaviour.” Cardiometabolic health needs to be viewed on a multilevel, integrative basis to make causal inference (Figure 2). Over time, an intervention (e.g., food) that may produce an outcome of interest can be investigated for its biologically effective dose, biological effect, and impact on change in structure and function. Taking into account genetics and epigenetics can help improve measurements of exposure, mediators, modifiers, and outcomes, as well as predict outcomes or prognosis and identify targets and off-targets. These can all be used to personalise dietary interventions.
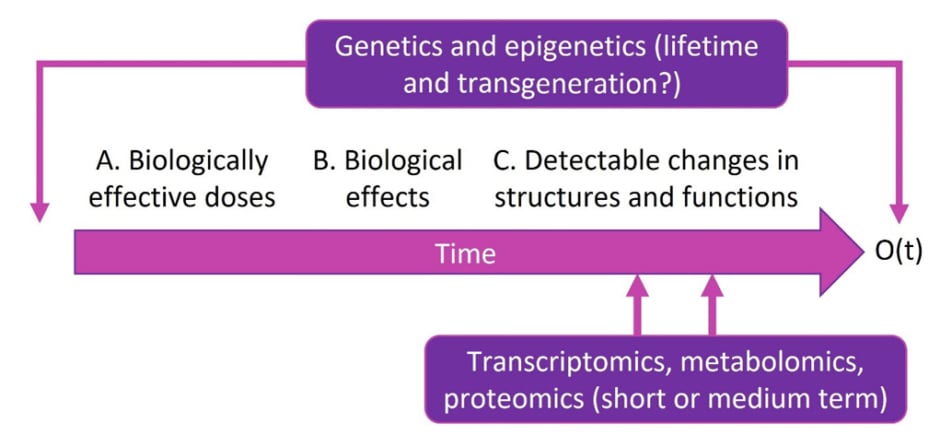
Figure 2: An integrative framework to evaluate causal relationships in diverse populations.
Reproduced with permission from Simin Liu, who retains its copyright.
O(t): outcome over time
Gene–Diet Interaction
Using genetic analysis that takes all of these factors into account, it is now possible to identify some of the key regulatory drivers to explain different phenotypes in T2D and CVD.37 For instance, TRPM6 and TRPM7 are genes that encode proteins involved in magnesium transport and were identified to be potential predictors of the risk of T2D after analysis, following magnesium supplementation in people who were low in this element.38
Conclusions
An integrated framework is needed that can enhance study design, analysis, and interpretation and allow for identification of effective prevention and intervention strategies at each phase of the life cycle, taking into account the concept of interaction.
Prevention of diseases such as T2D require definition of biological mechanisms and triggers. This can be helped by the use of biomarkers.
Nutritional Interventions for Addressing the Interaction Between Diabetes and Sarcopenia
Philip Atherton
Lean body mass (LBM), including skeletal muscle, is continuously being remodelled throughout the day by the simultaneous process of protein synthesis and protein breakdown (turnover), also known as postprandial anabolism.39 Researchers have shown that the timing and type of protein source can impact the rate of muscle hypertrophy and atrophy.40
The Actions of Protein on Insulin and Incretin Hormones
To further elucidate this concept, recent work by Atherton and colleagues showed that after approximately 45 minutes following protein consumption there is an insulin spike, even when carbohydrates are not consumed,41 which is associated with anticatabolism.42 Furthermore, in another study they carried out wherein participants ingested an EAA oral mixture, they observed an increase in GLP-1 and glucose-dependent insulinotropic polypeptide,43 suggesting, according to Atherton, that “the insulin response we see is at least partially driven by an incretin response following essential amino acid administration.”
Protein and Essential Amino Acid Supplementation in Older People
Maintenance of habitual protein intake is especially important in older individuals44 as sarcopenia, defined as a reduction in muscle mass and function that can drive frailty, is more likely to occur.45 A trial investigating effects of EAA supplementation on elderly participants with sarcopenia showed use of EAA supplementation for up to 16 months can help offset decline in LBM.46 Moreover, a similar study showed that EAA supplementation was associated with an increase in muscle synthesis and muscle insulin-like growth factor-1.47
Age-related skeletal muscle decline is especially evident in people with T2D.48 One trial showed that people with T2D who did not consume adequate amounts of protein had a significantly higher mean number of functional limitations and, overall, poorer diet quality.49 This was found to be particularly true among people with insulin resistance who were given EAA supplementation and showed an improvement in functional measures such as walking speed and balance tests.50 Supplementation with just L-arginine, combined with exercise, in T2D has also been shown to improve fat-free mass and glucose tolerance.51
Atherton and colleagues hypothesised that targeting incretin hormones with nutrition could help rescue protein anabolism in elderly people. To test this, they infused GLP-1 into a group of males (mean±standard error of the mean age: 71±1 years) and showed a marked increase in microvascular blood flow following a meal, which was greater in the presence of GLP-1. In other words, “targeting GLP-1 in subjects with T2D might also have a muscle preserving function.”52
Conclusions
Sarcopenia is an issue of concern in older people as well as older adults with T2D, which may be helped with sufficient protein and EAA intake.
Novel nutrient approaches might impact muscle outcomes via incretin effects.
β-hydroxy-β-methylbutyric Acid, Lysine, and Arginine: A Combination of Ingredients that Ameliorates Diabetes Muscle Loss and Its Complications in Streptozotocin‐Induced Rats with Diabetes
Jose-María López-Pedrosa
Rats with Diabetes Model to Investigate Supplementation Effects
López-Pedrosa presented his group’s study findings that evaluated the effects of a combination of dietary components, each shown to have a physiological effect, on skeletal muscle loss and metabolic changes in a diabetic rat model. This combination included SDC (which can decrease postprandial glycaemic and insulinemic responses),53 HMB (which has been shown to increase muscle mass),54 arginine (as discussed above),51 and lysine (that can lower postprandial glucose and insulin responses).55 T2D was induced in 12-week-old male Wistar rats via a high-fat diet and administration of a β-cell mass-reducing chemical agent (data on file).
The mice in the supplemental diet group, compared with mice on the high-fat diet, showed significantly less decline in muscle mass following the diabetes-inducing period along with a significant increase in expression of skeletal muscle glucose transporter 4. While there was an increase in FPG for both groups, it was significantly higher in the mice on the standard diet. Furthermore, the mice in the standard diet group experienced a decrease in fasting insulin levels, while those in the supplemental diet group had a significant increase. Overall, both groups showed a decrease in insulin sensitivity, but to a significantly lower degree in the supplemental group.
Additionally, while both groups experienced an increase in HbA1c, it was higher in the standard diet group, and the mice on the standard diet showed a slight increase in serum triglyceride/high-density lipoprotein:choline ratio, which is proposed to be a predictor of cardiovascular outcomes.28
Finally, isolation and analysis of pancreatic β-cells showed a significant increase in insulin production when exposed to a combination of HMB, lysine, and arginine only, suggesting this outcome is not dependent on SDC.
Conclusions
Supplementation with a mixture of SDC, HMB, arginine, and lysine, can preserve muscle mass and improve insulin resistance. Such supplementation might delay metabolic complications associated with diabetes progression.