Meeting Summary
Type 2 diabetes mellitus (T2DM) currently affects >8% of the world population. It is the leading cause of blindness, end-stage kidney disease, and neuropathy, and doubles the risk of developing cardiovascular disease. Until recently, the treatment of diabetes had broadly emphasised the management of hyperglycaemia as the key diagnostic criterion for T2DM. The pathophysiology of T2DM however is now understood to be rooted in the associated metabolic syndrome including intra-abdominal fat deposition, lipid abnormalities, high blood pressure, hypercoagulability, and macrovascular complications occurring in parallel with glucose dysregulation. Accordingly, closer attention to the medical management of these conditions is at the forefront of diabetologists’ treatment rationale in an attempt to prevent and mitigate both micro and macrovascular complications, especially in light of the recent positive data from cardiovascular outcome trials with both sodium-glucose co-transporter 2 (SGLT2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists. This symposium included a discussion of the evolution of treatment for T2DM and presented the rationale for the use of novel agents and combination therapies for patients according to their individual disease progression. Several newer drug classes were highlighted, including GLP-1 receptor agonists, dipeptidyl-peptidase-4 inhibitors (DPP-4 inhibitors), and SGLT2 inhibitors. Finally, an overview of the exciting new fields of prevention and treatment for T2DM were discussed; including stem cell proliferation into pancreatic beta cells, the reprogramming of white adipose tissue into brown fat, mimicking physiological effects of bariatric surgery pharmacologically, and other approaches to make the treatment more targeted and personalised.
Introduction
T2DM currently affects >422 million individuals (>8% of the world population), representing 90% of the total diagnosed cases of diabetes.1 Traditionally, T2DM has more severely affected economically developed countries with increasing rates of risk factors such as high obesity, low exercise levels, tobacco smoking, and a large elderly population. More recently however, an increased prevalence of T2DM has become obvious in mid and low-income regions.1
T2DM is characterised by inadequate insulin secretion from the beta islets of the pancreas on the background of insulin resistance.2,3 Complications of diabetes include: diabetic emergencies, such as ketoacidosis, hypoglycaemia, and hyperglycaemic hyperosmolar states;4 microvascular complications such as nephropathy and retinopathy leading to renal failure and blindness, respectively; macrovascular complications such as ischaemic heart disease and stroke;5 increased susceptibility to infection;6 and cognitive dysfunction arising from a higher incidence of vascular dementia and Alzheimer’s disease.7
Before the importance of hypertension and lipid control were fully recognised, the treatment of diabetes almost exclusively revolved around the management of hyperglycaemia and the reduction in lifestyle risk factors. Treatment targets for glycaemic control were established using a series of long-term trials.8,9 Intensive glycaemic control for over 11 and 16 years can prevent macro and microvascular complications, respectively.10 However, many of the larger trials have not shown a significant association between intensive glycaemic control and prevention of cardiovascular complications.11
A greater understanding of the pathophysiology of T2DM in the context of broader metabolic syndrome (Figure 1) has opened up a range of alternative treatment options. In the last 10 years, a number of novel glucose-lowering medications have been approved for clinical use. Several entirely new classes have been developed, including the GLP-1 receptor agonists, the DPP-4 inhibitors, and the SGLT2 inhibitors. When used in combination with conventional glycaemic control options, early trial data appear to show marked reductions in both macro and microvascular complications, as well as mortality rates.
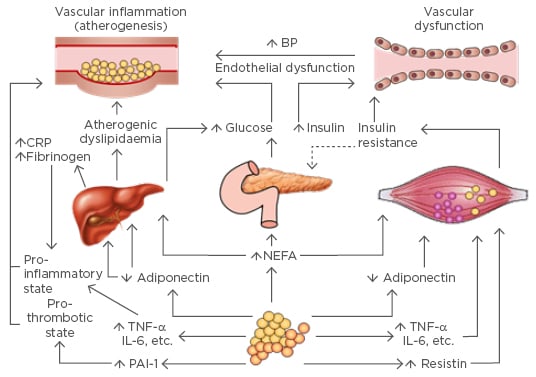
Figure 1: Pathological mechanisms and effects on end-organ systems in Type 2 diabetes mellitus in the context of metabolic syndrome.
BP: blood pressure; CRP: C-reactive protein; IL: interleukin; NEFA: non-esterified fatty acid; PAI-1: plasminogen activator inhibitor-1; TNF-α: tumour necrosis factor-α.
Adapted with permission from Grundy SM, 2006.12
The introduction of new agents into the market is heavily influenced by the burgeoning number of patients living with diabetes and the impact of treatment costs on health budgets. However, it is very important to emphasise that T2DM is rooted in metabolic syndrome, in patients with intra-abdominal fat deposition, lipid abnormalities, high blood pressure, hypercoagulability, and macrovascular complications that present prior to hyperglycaemia. The purpose of this symposium was to discuss the current state of evidence concerning agents that can act on a broader range of pathophysiological manifestations of metabolic syndrome rather than glucose control alone, in order to clarify therapeutic choices based upon personalised care.
Type 2 Diabetes Mellitus: Is it Time to Shift the Focus Away from Glycaemic Control?
Professor Chantal Mathieu and Doctor Juris Meier
Key Points
- T2DM used to be considered a disease of aberrant glucose control by pancreatic beta cells
- It is now understood to have a more complex pathophysiology within the context of metabolic syndrome
- Diabetes can be complicated by both macro and microvascular effects leading to early morbidity and mortality
- Newer agents that can regulate glucose with low incidence of hypoglycaemia and reduce blood pressure and weight could herald a new age of treatment for T2DM
All too often, diabetes treatment is reduced to glycaemic control with the aim of lowering associated complications yet in order to really affect the outcome in people with T2DM, broader interventions may be necessary to really affect this complex disease and its comorbidities. T2DM is a disease of chronic hyperglycaemia; a high glucose concentration is the specific diagnostic criteria for diabetes. However, individuals manifest changes indicative of macrovascular damage long before they develop overt glycaemic abnormalities and are diagnosed with T2DM,13 suggesting that clinicians should be considering the wider impact of metabolic syndrome as a clinical imperative. The issue is not entirely clear cut because although it is indisputable that obesity increases the risk of diabetes,14 a longitudinal study conducted over 7 years in almost 3,000 individuals aged ~55-years demonstrated that only 20% of individuals with combined obesity and metabolic syndrome will eventually develop T2DM.15 In this population, the increased risk is likely to be the result of an increased insulin demand of >3-fold, compared with lean individuals.16 Once diabetes is diagnosed, the relative risk of cardiovascular events increases in a linear manner for every 1% increase in glycated haemoglobin (HbA1c).17
The progressive decline in pancreatic beta cell function is clearly the key pathological step once the hyperglycaemic state of T2DM becomes clinically evident. A reduced beta cell mass causes aberrant and reduced insulin pulsatility, leading to the triple effect of reduced insulin signalling, alpha cell dysfunction, and reduced glucose uptake by skeletal muscle and adipose cells.18 These effects combine with insulin resistance to cause hyperglycaemia.
The UKPDS is the largest prospective study of newly diagnosed patients with diabetes and has been reporting its findings from 3,867 newly diagnosed patients since its inception in the 1970s, in terms of tight glucose control (by sulphonylurea or insulin and metformin in an obese subgroup) or dietary control alone.10 The UKPDS showed a 25% reduction in microvascular events with tight glycaemic control over 10 years, without a statistically significant impact on macrovascular events, despite a relative risk reduction of 16% between the intensive control and standard treatment groups.19 The interpretation of these results implies that the substandard management of other aspects of metabolic syndrome, including blood pressure control, 40–50 years ago could have accounted for the lack of clinical benefit in this regard.
Event rates for macrovascular complications of T2DM (most commonly reported as myocardial infarction, stroke, and peripheral vascular disease) have reduced substantially over the past 20 years, not only due to improvements in glucose levels, but also improved lipid and blood pressure control.20 For example 10 years ago, the prospective ADVANCE randomised controlled trial (RCT) assessed the combination of intensive glucose lowering and intensive blood pressure control approaches versus standard glucose lowering therapy in >11,000 patients worldwide.21 The intensive group showed a 14% reduction in microvascular complications,22,23 primarily as a result of reduced rates of nephropathy,24 and the benefit to the individual patient far exceeded the risks of adverse effects such as hypoglycaemic episodes.25
In a focussed study, the HOPE trial assessed the reduction of blood pressure in a general population of individuals at high risk of developing cardiovascular disease, with a subgroup of ~3,500 patients with T2DM.26 The trial was stopped early because of the clear benefits of the use of the ACE inhibitor, ramipril, on reducing cardiovascular events particularly within the diabetic subgroup (a 25% overall reduction in the primary endpoints of cardiovascular death, non-fatal myocardial infarction, and stroke). The HOPE study also showed a reduction in the rate of nephropathy in patients with T2DM who received an ACE inhibitor, in line with findings from the ADVANCE study.27 Ramipril also reduced the rate of diagnoses of diabetes in the general population. A health economics assessment of the use of ramipril showed it to be an economically attractive agent with major clinical effects in a series of trials related to diabetes.28 Improved mortality rates and clinical outcomes in terms of cardiovascular events, coronary heart disease, and stroke, have also been shown in diabetic subgroups of larger meta-analyses among patients treated with antihypertensives.29-31
Abnormalities in lipid metabolism as part of metabolic syndrome play a key role in dysglycaemic states and could increase the risk of complications in diabetes. With regards to lipid control, several meta-analyses have assessed the efficacy of lipid-lowering agents on complications related to T2DM.32 Early studies on atorvastatin through the CARDS trial initially showed a very strong association between statin use and reduced cardiovascular events in patients with T2DM.33,34 The effect persisted at low-dose treatment (10 mg atorvastatin) for all primary endpoints, including acute coronary events, coronary bypass grafting, and stroke, and the secondary endpoint of any cardiovascular event (0.68, 0.55, and 0.85, respectively; 95% confidence interval [CI]), while a reduction in death from any cause also almost reached significance in this study (hazard ratio: 0.73, 95% CI: 0.52–1.01, p=0.059).35 Notably, the FIELD study also showed reduced rates of microvascular complications and retinopathy in particular, alongside reduced serum cholesterol and triglyceride levels in patients who received the cholesterol-reducing drug fenofibrate.36
The Steno-2 study from Denmark elegantly examined the prospective use of multiple drug interventions along with behaviour modelling for a small number of patients (n=160) with T2DM. The study determined a 20% reduction in the number of cardiovascular disease events with intensive control of all metabolic syndrome parameters. Microvascular complications were also significantly reduced in the intensive control group, with relative risk reductions of 61% for nephropathy, 58% for retinopathy, and 63% for neuropathy. These findings persisted for >4 years after the 8-year study period37 and patients in the intensive control group lived for 7.9 years longer than those in the control.38 Further long-term results in these patients are awaited eagerly, but give a tantalising glimpse of the future potential of intensive treatment to achieve combined blood pressure, lipid, and glycaemic control to offset complications of T2DM.38,39
However, the clinical difficulties of patient tolerance, side effect profiles, and the expense of prescribing a polypharmacy of medications are important barriers to achieving the paradigm described in the Steno-2 study even though combined treatment for hypertension, coagulopathies, and dyslipidaemia constitutes the mainstream therapeutic approach nowadays. Importantly, new agents that have a range of associated positive effects in patients with T2DM are going through clinical trials at present with promising results. These new classes include the SGLT2 inhibitors, GLP-1 receptor agonists, and the DPP-4 inhibitors. SGLT2 inhibitors such as dapagliflozin, the first to be introduced in Europe, canagliflozin, and empagliflozin, block the activity of the SGLT2 transporter in the proximal tubule, preventing up to 90% of the reabsorption of glucose.40 The resulting natriuresis and fluid loss mean that blood pressure is lowered as well, which is an additional benefit for most patients with T2DM. The EMPA-REG outcome study demonstrated that treatment with the SGLT2 inhibitor empagliflozin, compared with a placebo, reduced death rates due to cardiovascular events within only 3 months as well as microvascular events, mainly driven by nephropathy within 6 months.41 Empagliflozin also demonstrated a 35% reduction in hospitalisation for heart failure. Given that cardiovascular risk reduction occurred so soon after initiation of the trial, the results are likely to arise from osmotic diuresis or an unknown direct cardiac effect, rather than reductions in blood glucose levels, body weight, and blood pressure. Furthermore, the risk of hypoglycaemic events with SGLT2 inhibitors has been found to be low, although more work is needed to assess the tolerance of these agents in elderly or frail populations with T2DM.
The incretin system comprises a pair of metabolic hormones that act to reduce blood glucose levels by stimulating the activity of pancreatic beta cells. GLP-1 receptor agonists, such as the injectable drugs exenatide and liraglutide, stimulate insulin secretion from beta cells, but are associated with a lower rate of hypoglycaemic events compared with insulins, because their effects are glucose-dependent. In the Phase III LEAD series of trials, the long-acting GLP-1 receptor agonist liraglutide was shown to provide glycaemic control, weight reduction, and systolic blood pressure control, with improved renal outcomes within 1 year, and a 22% reduction in mortality.42-44 The LEADER trial determined a 22% risk reduction for death from cardiovascular causes, non-fatal myocardial infarction, or stroke, in patients given liraglutide compared with those receiving placebo.45 The EXSCEL study was established to determine whether exenatide, in addition to the patient’s usual care regime for T2DM, could reduce the risk of cardiac disease.46 Early results expected in 2017 will demonstrate whether long-acting exenatide has primary and/or secondary prevention effects for cardiovascular outcomes in T2DM.
Finally, DPP-4 inhibitors, also called gliptins, act to block DPP-4, resulting in increased levels of incretin system hormones; there are now 11 drugs within this class, some of which are still pending regulatory approval. A meta-analysis of the use of DPP-4 inhibitors in patients with T2DM showed no effect on cardiovascular mortality or stroke, or even all-cause mortality but suggested an increased risk of heart failure (risk ratio: 1.16; CI: 1.01–1.33).47
There is no doubt that glycaemic control is important however there appears to be a differential effect depending on when glycaemic control is administered because treatment appears to be more beneficial when administered early in the course of the disease. Although the microvascular, macrovascular, and mortality benefits of novel treatment therapies, such as SGLT2 inhibitors and GLP-1 receptor agonists, support their use as antihyperglycaemic agents, more evidence is required before using these therapies to specifically target the complications of T2DM. In addition, intensive control is associated with a higher risk of severe hypoglycaemia and weight gain, which are the two key barriers to effective long-term glycaemic control. Consensus was reached during the debate that there needs to be a change in the treatment paradigm, whereby newer therapies, in particular SGLT2 inhibitors, should be elevated in the hierarchy of treatment modalities in light of their efficacy at controlling glycaemia and positive effects on cardiovascular risk.
This part of the symposium concluded with the acknowledgement that new single agents with multiple effects would be likely to be more clinically acceptable than multiple treatments (e.g. statin + sulphonylurea + insulin, or a DPP-4 inhibitor + statin + ACE inhibitor, etc.). The final point made in the discussion was that there was less broad evidence from prospective RCTs to go on supporting the use of metformin, sulphonylureas, and insulin, and yet these were the most frequently used drugs, a point causing some discussion within the audience.
The Chair, Prof Baptist Gallwitz, then summarised the key points of the debate: i) glycaemic control remains an important risk factor in the management of micro and macrovascular complications and mortality in patients with T2DM; ii) novel treatment therapies such as SGLT2 inhibitors and GLP-1 receptor agonists have microvascular, macrovascular, and mortality benefits supporting their use in everyday treatment; iii) we need more evidence before using these novel therapies to specifically target the complications of T2DM but studies are ongoing; and iv) we may need individualised treatments tailored for the early and late stages of diabetes.
Combination Therapy in Type 2 Diabetes Mellitus: Why, What, and When?
Professor Chantal Mathieu
Key Points
- T2DM is a complex, multifactorial disease characterised by the progressive decline of pancreatic beta cell function requiring more intensive therapy over time
- Antihyperglycaemic therapy therefore evolves over the disease course from single agent therapies to combinations of three or more agents
- A physiological basis exists for combining therapies with complementary mechanisms of action that help to control hyperglycaemia with associated clinical benefits
- Combination therapy should be used earlier to maintain adequate glycaemic control
Figure 2 illustrates the general recommendations for the treatment of T2DM according to the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Prof Mathieu went on to present the history and recent trial data to support the use of dual and triple therapies for the treatment of T2DM, focussing on the current state of knowledge of the different underlying pathological mechanisms of T2DM and the drug targets that are known against each aspect (Figure 3). T2DM is a complex and multifactorial disease with multiple physiological abnormalities including increased endogenous glucose production, increased glucagon secretion, impaired insulin secretion, decreased incretin effects, increased lipolysis, and increased glucose reabsorption. Therefore, a rationale exists for using combination therapies to target multiple physiological abnormalities simultaneously to improve patient outcomes in the context of declining pancreatic beta cell function.
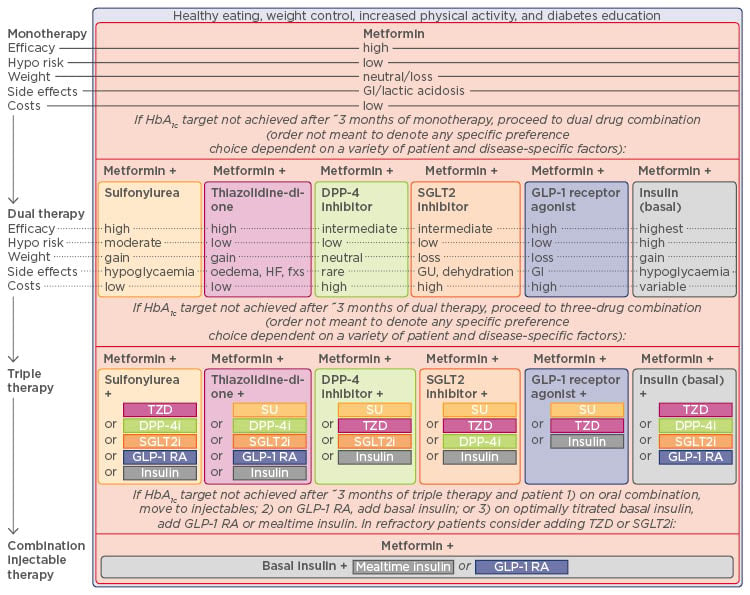
Figure 2: Recommendations for Type 2 diabetes mellitus antihyperglycaemic therapy.
ADA: American Diabetes Association; DPP-4i: dipeptidyl peptidase-4 inhibitor; GI: gastrointestinal; GLP-1 RA: glucagon-like peptide-1 receptor agonist; GU: genitourinary; SGLT2i: sodium-glucose co-transporter 2 inhibitor; SU: sulphonylurea; TZD: thiazolidinedione; HbA1c: glycated haemoglobin; HF: heart failure.
Published with consent from Inzucchi et al., 2015.48
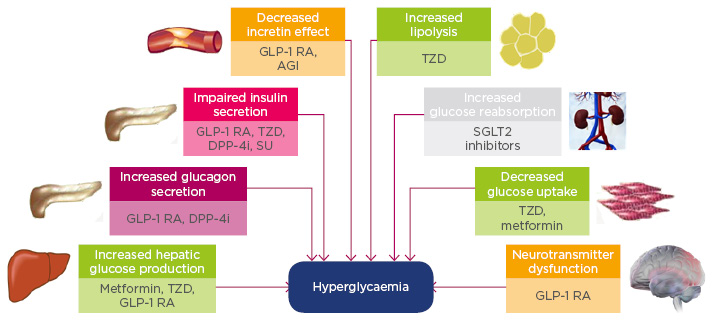
Figure 3: Pathogenesis of Type 2 diabetes mellitus and the site of action of antihyperglycaemic agents.
AGI: alpha-glucosidase inhibitor; DPP-4i: dipeptidyl peptidase-4 inhibitor; GLP-1 RA: glucagon-like peptide-1 receptor agonist; SGLT2: sodium-glucose co-transporter 2; SU: sulphonylurea; TZD: thiazolidinedione.
Metformin may work as a GLP-1 enhancer and sensitiser49 and indeed combining incretin-related therapies with metformin is associated with improved outcomes. The ADA/EASD position statement referred to in Figure 2 does not contain recommendations for the optimal second-line agent, but some trial evidence has revealed different advantages that might yield personalised approaches to therapy when more data become available. For example, the addition of DPP-4 inhibitors to metformin improves glycaemic control, brings a lower risk of hypoglycaemia than some other therapies, and prevents weight gain in patients with T2DM compared with glipizide.50 Furthermore, the combination of a SGLT2 inhibitor with metformin enhances the glucose-lowering effects of metformin alone as well as sustained reductions in HbA1c, systolic blood pressure, and body weight.50
When two agents are unable to achieve glycaemic control, a third agent is required; there is a wide range of possible options (Figure 2). Triple oral combination therapies with metformin, a SGLT2 inhibitor, and a DPP-4 inhibitor (saxagliptin and dapagliflozin) were associated with improved outcomes compared to either dual therapy.51,52 The DURATION-8 clinical trial51 reported a significant reduction in HbA1c levels, weight, and systolic blood pressure when these drugs were used in combination versus either medicine alone. Treatment with metformin, along with a combined injection of insulin and a GLP-1 receptor agonist, is associated with improved glycaemic control compared with any of the individual components.53
Prompt intensification of therapy leads to improved glycaemic control and early evidence suggests the use of combination therapy should reduce the incidence of long-term complications. Glycaemic control is still important, but no longer the sole focus of treatment. Persuading clinicians to adopt such strategies will be the next challenge for leaders within the field.
Where Do Novel Therapies Best Fit Within the Treatment Paradigm?
Panel Discussion with Audience Participation
The panel discussion opened with the consensus that novel agents have led to openings for the treatment of patients who previously would have had no realistic treatment options. Newer agents have been developed to target specific molecular mechanisms than older medications, such as sulphonylureas. Agents such as GLP-1 receptor agonists and SGLT2 inhibitors have fewer off-target effects, rarely produce hypoglycaemic events, and can assist with weight management and blood pressure control as well as glycaemic control. The selection of which medication amongst the ‘explosion’ of novel agents should be used in individual management plans, and the consequent development of personalised approaches for the treatment of T2DM, is now the focus of intense debate and research.
For the three novel classes of medication, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors, RCTs show a reduction in HbA1c of 0.6–0.9%, 1–1.2%, and 1–1.5%, respectively. However, it is important to show that RCT data can be transferred into meaningful data from real-world clinical practice, and should be based on criteria other than glycaemic control. Dapagliflozin has been shown in RCTs to reduce the HbA1c in patients with T2DM by 1% with weight loss either as a single agent,54 or when combined with metformin of between 1.5 kg and 3 kg.55 In a cohort study conducted using a general practice database (Clinical Practice Research Datalink [CPRD] study), these changes were reproduced, which is reassuring from the point of view of the clinical efficacy of this SGLT2 inhibitor. Similarly, a comparison of saxagliptin (a DPP-4 inhibitor) and metformin versus dapagliflozin and metformin showed that dapagliflozin was better than saxagliptin at reducing weight, in as much as saxagliptin seemed to have no effect on weight although it was effective for glycaemic control in terms of HbA1c reductions.56
In regards to the comparison between GLP-1 receptor agonists and DPP-4 inhibitors, both drug classes act on the incretin system but on different targets. GLP-1 receptor agonists result in a larger reduction in HbA1c than DPP-4 inhibitors. DPP-4 inhibitors are also neutral with regards to weight, whereas GLP-1 receptor agonists can lead to a weight loss of almost 2 kg.41 When the symposium audience was asked what their opinion was regarding which class of drug was most appropriate in patients with no history of cardiovascular disease who were inadequately controlled on metformin, almost 50% voted for SGLT2 inhibitors, 25% for GLP-1 receptor agonists, 20% for DPP-4 inhibitors, and <5% for sulphonylureas and other older drug classes. Although this was perceived as encouraging by the panel, the truth is that there is still a strong phenomenon of treatment inertia in clinicians treating patients with T2DM that should be addressed. This phenomenon most likely reflects the budgetary constraints of healthcare systems across different countries as well as the fact that injectable agents were less likely to be accepted by patients than oral formulations.
The gap between real-world and trial experience was emphasised in the study groups assessed in some of the key published trials. For example, most clinicians would institute advice on lifestyle changes and metformin if the HbA1c level was >6.7% in an individual but some of the RCTs that looked at new agents used groups of patients with untreated diabetes and HbA1c levels >8.5%. The point was emphasised that the chance of a single agent being able to reduce the HbA1c to the target range if the starting point was high, was much lower than early treatment, whereas a patient is more likely to achieve their HbA1c target if treated early before an uncontrolled level is reached. To this end, prompt combination therapy should be instigated to give the best chance of preventing complications of T2DM. Trials should also reflect real-world situations as closely as possible, which is not always the case.
The audience was asked which side effects they were most concerned about with the use of GLP-1 receptor agonists or SGLT2 inhibitors. However, the main concern of increased rates of urinary tract or genital infections are only a feature of the use of SGLT2 inhibitors. However, as genital infections infrequently lead to serious consequences and the incidence of urinary tract infections has not been noted as increased in trial data, these concerns were largely allayed by the panel. The panel members were more concerned about the rate of one case of ketoacidosis in every 1,000 patients taking SGLT2 inhibitors.
The discussion then turned to the safety of GLP-1 receptor agonists. When questioned about specific adverse effects, almost half of the audience responded that pancreatitis was a major issue. An unpublished meta-analysis of the principal trials of this drug class and cardiovascular risk in T2DM (ELIXA and LEADER) as well as three trials that assessed DPP-4 inhibitors, showed an odds ratio of 1.8 for pancreatitis, for DPP-4 inhibitors versus placebo. In terms of the data available for GLP-1 receptor agonists, 2,000 patients would need to be treated for 1 year to elicit one event of pancreatitis, which should be put in context against other more frequently used medications such as azathioprine with a pancreatitis frequency of 2–5% per year. The early studies that showed pancreatitis might have been a larger problem than later studies reflected have obviously influenced clinician prescribing practices, as reflected by the 45% of the audience that considered it to be a principal consideration in prescribing GLP-1 receptor agonists. One of the further unanswered questions regarding the use of GLP-1 receptor agonists is the clinical significance of the slight increase in gallbladder disease and whether this was the result of weight loss or a direct effect of the drug on gallbladder motility.
Given the side effects of new oral agents and the nausea associated with the GLP-1 receptor agonists, the panel discussed that patient tolerance for these drugs may not be high over the longer-term. The clinician however, might be able to actively guide second-line drug selection once metformin starts to fail based on the clinical presentation of the patient outside of their glycaemic control. For example, clinical trial data for SGLT2 inhibitors show that they produce osmotic diuresis, resulting in volume depletion. Therefore, for a patient showing early signs of cardiac failure, this would probably be the best choice as a second-line agent. Similarly, for patients with atherosclerosis, GLP-1 receptor agonists might be the best option in light of their effects in reducing plaque size. The consensus amongst the panel was that the choice of drug should be guided by clinical acumen, awareness of the relative benefits of each second-line agent, and an avoidance of drugs that are likely to cause hypoglycaemic episodes.
Managing our Patients Beyond the Beta Cell
Doctor Marcus Schindler
Finally, the role of preventative medicine was acknowledged as being one of the key future interventions for T2DM allowing clinicians to intervene before vascular changes start to occur. The most likely approaches were proposed to be mimicking effects of bariatric surgery pharmacologically, potentially using more therapies that target the incretin system such as the GLP-1-glucagon co-agonists under development by several companies as well as combination therapies that will use cheaper generic options to address all of the risk factors for diabetic complications. Diabetes prevention is a hugely active field with many developments and ongoing studies of novel treatments. There are multiple other preclinical and early stage trials ongoing for alternative approaches to disease treatment and prevention that are expected to change the treatment paradigm for T2DM. These include pancreatic islet cell production from stem cells, the prevention of pancreatic beta cell dedifferentiation, enhancing mechanisms to increase beta cell proliferation, and increasing brown/beige adipocyte mass. Underpinning all of these advances will be the continued basic science research to delineate pathways, and genetic analyses that will enable precision medicine such as the next wave of insights into microRNA signalling. MicroRNA heterogeneity may be one of the leading ways that precision medicine can advance in T2DM.
Conclusions
In summary, the management of T2DM has come a long way over the last 20 years with a range of new therapeutic options providing greater opportunities for individualised care. Glycaemic control remains the central pillar of therapy; maintenance of blood glucose within a tight target range has well-established benefits on microvascular disease and if achieved early, the potential for macrovascular benefit over the long-term. However, an understanding of the underlying pathophysiology of T2DM, particularly cardio-renal-metabolic interplay, can allow a more rational approach to management. Newer therapies may be used to target specific physiological dysfunctions, maximising the overall benefit to the patient in terms of body weight, adverse effects, and the progression of micro and macrovascular disease. Furthermore, the appropriate choice and timing of combination therapy can optimise care for the individual patient rather than aiming for blanket targets.
With the range of therapies now on offer and the potential of newer agents in development, we may finally be turning a corner in the management of T2DM with the chance of making lasting and fundamental improvements to patient care.