Meeting Summary
Type 2 diabetes mellitus (T2DM) and non-alcoholic fatty liver disease (NAFLD), which includes non-alcoholic steatohepatitis (NASH), are highly prevalent diseases associated with an increased risk of morbidity and mortality. Obesity/overweight and dietary intake are important risk factors. Individuals with T2DM are at increased risk of death even in the setting of good glycaemic control. This highlights the importance of preventing T2DM altogether, but also utilising nutritional strategies in individuals with T2DM to help optimise glycaemic control and possibly achieve T2DM remission. Furthermore, current evidence suggests that a weight loss of 15% or more increases the likelihood of remission. Jamy Ard focused on the benefits of meal replacements and highlighted data demonstrating that a meal replacement programme can promote increased weight loss and increased rates of T2DM remission compared with other strategies. The key predictor of morbidity and mortality in NAFLD and NASH is liver fibrosis. Moderate (8–10%) total body weight loss can significantly improve liver histology. Wajahat Mehal discussed sustained weight loss and improved liver health and focused on meal replacements and very-low-calorie diets (VLCD) such as OPTIFAST® for the management of NAFLD/NASH.
Meal Replacements as Part of a Weight Management Strategy for Improved Glucose Control and Diabetes Remission
Jamy Ard
Nutrition in Type 2 Diabetes Mellitus
Ard opened his talk by discussing the role of nutrition in T2DM. For some individuals, T2DM is a consequence of excess weight gain. The onset and severity of T2DM is further complicated by diet quality.1 Nutrition therapy has always been a mainstay of T2DM management;2 however, the effectiveness of anti-diabetes pharmacological therapies has somewhat relegated the importance of nutrition, with consequences including greater need for medication, higher disease burden, higher risk of side effects and adverse events, and increased weight gain.
The link between T2DM and overweight/obesity has been clearly established, with 87.5% of individuals with T2DM in the USA having overweight or obesity (BMI>25 kg/m2).3 Furthermore, a positive correlation between the age-adjusted prevalence of obesity and that of diabetes was demonstrated in the USA between 1994 and 2012.4 Poor diet quality, energy imbalance, and increased adiposity promote an inflammatory response in the adipose tissue, which leads to insulin resistance. This results in increased glucose production by the liver, altered glucose uptake by the muscle, and dysglycaemia.5
Ard highlighted the importance of nutrition and diet in reducing the risk of developing T2DM. Diets rich in red meat, white rice, and sugar-sweetened beverages are associated with an increased risk of T2DM, while greater consumption of leafy green vegetables, dairy, wholegrains, and avoiding sugar-sweetened beverages are associated with a lower risk of T2DM.6 Nutrition is also critical for the management of patients who already have T2DM by promoting a healthy dietary pattern to help improve glycaemic and weight control.2 However, even in the setting of good glycaemic control, individuals with T2DM are at increased risk of death either from any cause or specifically from cardiovascular disease compared to those without T2DM.7 This highlights the importance of preventing T2DM altogether, but also utilising nutritional strategies in individuals with T2DM to help optimise glycaemic control and possibly achieve T2DM remission.
Diabetes Remission
Diabetes remission is defined in a consensus paper as achieving and maintaining HbA1c <6.5% for at least 3 months without the use of anti-diabetes therapy.8 The types of therapies that can lead to diabetes remission include bariatric surgery and intensive medical weight loss interventions. For example, individuals with T2DM experienced a median disease-free period of 8.3 years following Roux-en-Y gastric bypass (RYGB), which equates to a reduction in disease burden and medication use for a significant time period.9,10 Although not all individuals who undergo gastric bypass achieve remission, a number of predictors of response have been established, including younger age, shorter duration of diabetes (<8 years), not receiving insulin at the time of surgery, and greater weight loss.11 The Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial showed surgical treatments were superior to intensive medical therapy and RYGB outperformed sleeve gastrectomy at 3 years and 5 years in terms of the proportion of individuals who achieved diabetes remission.12 Ard highlighted that importantly, almost one-third of individuals with T2DM achieve sustained remission of diabetes 5 years following RYGB.
Lifestyle intervention can also be associated with achieving diabetes remission. In the Look AHEAD (Action for Health for Diabetes) study, the intensive lifestyle intervention group (i.e., weekly group and individual counselling in the first 6 months followed by three sessions per month for the second 6 months and twice-monthly contact and regular refresher group series) was significantly more likely to experience any remission of T2DM (partial or complete), with a prevalence of 11.5% during the first year and 7.3% at Year 4, compared with 2% for the diabetes support and education group at both time points.13
Meal Replacement
Ard noted that “using meal replacements can be a really important strategy to help people improve their weight management outcomes.” Meal replacements, typically provided as a liquid (i.e., shake or soup) or bar, are portion controlled and designed to replace a meal and provide nutrition comparable to that of a balanced meal. Meal replacements are derived from research on survival in conditions where solid food was not available (e.g., during space flight).14 Meal replacements are available in a variety of settings, including both as medical and over-the-counter treatments. However, it should be noted that regulation regarding nutritional minimal standards is variable, with the U.S. Food and Drug Administration (FDA) providing no regulation, and the European Commission providing detailed guidance on minimums and maximums for energy and macro- and micro-nutrients.15
Meal replacements provide a simple way for an individual to adhere to a restricted meal plan. Dietary adherence can be challenging as people increasingly eat out, consume larger portions, and consume food that is highly palatable, which also leads to a higher intake.16,17 To implement and maintain a calorie-restricted diet, individuals need to manage several variables including high dietary restraint, low disinhibition, nutritional literacy, and ongoing behavioural modification (e.g., self-monitoring, self-regulation). Thus, meal replacements can be used to help overcome some of these challenges. Data from a meta-analysis showed that one or two meal replacements per day in a partial meal replacement diet led to greater weight loss compared to a low-calorie, food-only diet (difference of 2.5 kg at 3 months and 2.6 kg at 12 months).18 In addition, individuals who consumed a larger number of meal replacements, when prescribed as part of a low-calorie diet, had greater weight loss compared to those who consumed fewer meal replacements in the Look AHEAD trial.19
Ard described a target weight reduction of 15% to increase the probability of achieving diabetes remission. The Diabetes Remission Clinical Trial (DiRECT), a randomised controlled trial of intensive medical weight loss with total diet replacement (825–853 kcal/day) versus standard of care in primary care offices in England and Scotland, showed that 46% of participants in the intervention group achieved diabetes remission and that this increased to 86% of individuals who lost ≥15 kg.20 The average weight loss at 1 year was 10 kg in the intervention group versus 1 kg in the control group. The 2-year results of the DiRECT trial showed that 36% of intervention participants versus 3% of control participants had achieved diabetes remission at 2 years and 64% of those who sustained a 10 kg weight loss achieved remission.21 Ard emphasised that “this is strong clinical trial evidence that achieving a weight loss of 15% is associated with a high probability of diabetes remission.”
The Doctor Referral of Overweight People to Low Energy total diet replacement Treatment (DROPLET) and OPTIWIN trials provide further evidence to support the use of meal replacement to drive weight loss.22,23 The DROPLET trial showed that individuals randomised to total diet replacement (810 kcal/day for 8 weeks with gradual transition back to a food-based diet) lost 10.7 kg compared to 3.1 kg for usual care.22 Furthermore, 45% of individuals in the meal replacement group lost ≥10% of their body weight. The OPTIWIN trial involved randomisation of 273 participants to either the OPTIFAST programme (≥800 kcal/day of total meal replacement) or a food-based diet (500–750 kcal/day reduction below estimated total energy expenditure).23 Both groups had 6 months of a weight reduction phase followed by 6 months of a maintenance phase, with both receiving weekly behavioural counselling to support weight management efforts. Individuals in the OPTIFAST programme achieved an average of 12.4% and 10.5% weight loss at 6 months and 1 year, respectively (Figure 1). In addition, the weight loss achieved with the OPTIFAST programme was almost double that achieved by patients on the food-based programme (12.4% versus 6% weight loss at 26 weeks and 10.5% versus 5.5% at 52 weeks; p<0.001). Forty-two percent of individuals on the OPTIFAST plan achieved ≥15% weight loss at 6 months (compared to 11% of individuals on a food-based diet), with 30% maintaining that weight loss at 1 year. Forty-four percent of individuals on the OPTIFAST plan maintained ≥10% of their weight loss at 1 year. Thus, the OPTIFAST programme was associated with higher proportions losing 5%, 10%, or 15% of initial weight compared to the food-based programme.
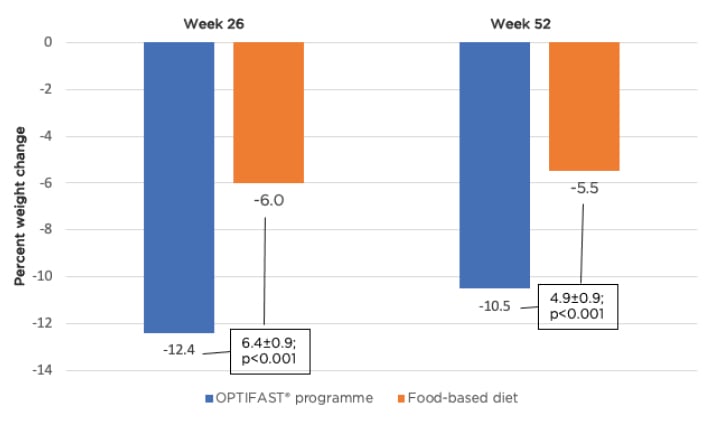
Figure 1: Percent change in weight at 26 and 52 weeks by treatment group.
Ard concluded that T2DM onset and severity is often associated with body weight and diet quality, and nutrition is an important tool for managing glycaemic control. Diabetes remission is considered achieved when the HbA1c <6.5% is maintained for at least 3 months without the use of anti-diabetes medications. Use of meal replacements with intensive behavioural weight management is shown to help individuals achieve the amount of weight loss necessary (i.e., target 15%) to improve chances of diabetes remission.
Sustained Weight Loss and Improved Liver Health: Meal Replacements and a Very-Low-Calorie Diet for the Management of Non-alcoholic Fatty Liver Disease
Wajahat Mehal
Pathophysiology and Epidemiology
Mehal opened his talk by defining NAFLD and NASH. NAFLD refers to steatosis in the liver where >5–10% of hepatocytes are fat-laden. NAFLD can progress to NASH, which is steatosis plus inflammation and hepatocyte injury/ballooning.24,25
It is well-understood how NAFLD can develop from obesity and excess nutrition, but it is less well-understood what drives the inflammatory process that occurs when NAFLD progresses to NASH. In the 1990s, Matzinger discussed “the possibility that the immune system does not care about self and non-self, that its primary driving force is the need to detect and protect against danger.”26 Cell death triggers the release of molecules normally contained inside cells; these molecules bind to various receptors outside the cell resulting in an inflammatory response. Mehal emphasised that “although inflammation after cell death is a general phenomenon in all organs of the body, for reasons that are not very clearly understood the amplitude of inflammation is much higher in the liver and therefore people present with NASH.”
The current prevalence of NAFLD in the USA is 30%, while the prevalence of NASH is 6%.27 In 2015, >80 million individuals in the USA had NAFLD, and this is projected to increase to almost 101 million by 2030.27 Approximately 1.3 million people had advanced fibrosis due to NASH in 2015; this is expected to increase three-fold by 2030 to almost 3.5 million.27 This is particularly important as fibrosis is responsible for most health issues in the NAFLD/NASH population.28,29 As a result of the growing prevalence of NASH, it is now estimated to have overtaken hepatitis C virus as the leading cause of hepatocellular carcinoma. Mehal highlighted that NAFLD is not restricted to older age groups and that many patients in their 20s and early 30s are diagnosed with this disease.30 In addition, first-degree relatives of patients with NAFLD-cirrhosis have a 12-times higher risk of developing advanced cirrhosis;31 this is likely to reflect a genetic element as well as the impact of a shared environment. A racial propensity is also evident with the highest prevalence of NASH observed in the Hispanic population and the lowest in African American people.29,32
Mehal explained that the majority of NAFLD/NASH seen in the clinic is subclinical, and patients generally do not have a very high BMI (32 kg/m2 in patients with NASH in clinical trials) and insulin resistance and metabolic syndrome are better predictors of risk. Although many patients can be identified by aspartate transaminase/alanine aminotransferase elevation, this has low sensitivity. Patients with elevated aspartate transaminase/alanine aminotransferases will usually be referred to a hepatologist; however, concerns remain over identification of patients with NAFLD/NASH with normal liver function tests. Many patients present with fibrosis/cirrhosis, but progression to fibrosis occurs in only 15–20% over 3–5 years. There is no effective tool available at present to identify patients with significant fibrosis.
Mehal emphasised that “it is not all about BMI,” and the presence of central obesity and features of metabolic syndrome (i.e., diabetes, hypertension, dyslipidaemia) are also helpful for diagnosis of NASH. Mehal noted a degree of symmetry in the relationship between diabetes, NASH, and obesity. For example, 60% and approximately 80% of individuals with T2D and NASH, respectively, have obesity. In addition, 37% of those with T2D have NASH and 44% of those with NASH have diabetes. A clear asymmetry, however, is observed in the prevalence of NASH (33%) compared to diabetes (12%) among those with obesity (i.e., almost three-fold higher).33 As a result of the strong association between NASH and metabolic syndrome, there has been a proposal to change the current nomenclature of NAFLD to metabolic dysfunction-associated fatty liver disease (MAFLD).34,35 A MAFLD diagnosis would require the detection of hepatic steatosis and at least one feature of overweight/obesity, T2DM, or metabolic dysregulation. A study showed that the prevalence of MAFLD was comparable to that of NAFLD and only 4.7% of participants met the diagnostic criteria for NAFLD but not MAFLD.36 Thus, Mehal noted that essentially the same population would be captured by either criteria; however, individuals with a low BMI without metabolic dysfunction would not be captured by the MAFLD diagnostic criteria.
The risk of liver-related morbidity and mortality increases exponentially with increasing fibrosis stage and patients with advanced fibrosis are at the greatest risk.28,37 In the USA, approximately one-fifth of the NASH population have Stage F3 or F4 fibrosis, while only 21% have no fibrosis.27 Mehal noted that “this is alarming as once patients develop fibrosis, it tends to progress.” At Stage F4 (i.e., cirrhosis), the disease state may not be fully reversible and for patients with F3, mitigating progression to cirrhosis is therefore a principal objective.38,39 Thus, urgent action is needed to prevent cirrhosis-related morbidity and mortality.40,41
Treatment and Prevention
Numerous pharmacotherapies are in development for NASH.42 However, even when drugs are approved, a better response is likely if weight can also be managed. Mehal noted that “pharmacological intervention and weight management will go hand in hand.”
A randomised controlled trial reported a significant decrease in intrahepatic triglyceride from 8.5% at baseline to 7.4%, 4.1%, and 3% with progressive weight loss of 5%, 11%, and 16%, respectively.43 Mehal emphasised that 11% weight loss is achievable with a low-calorie and very-low-calorie diet and moderate total body weight loss results in a proportionately much greater reduction in liver triglycerides. A further study examined the association between the magnitude of weight loss through lifestyle modifications and changes in histologic features of NASH.44 Improvement in NAFLD activity score was correlated with percentage of weight loss, and fibrosis stabilised in approximately 50% of patients with 7–10% weight loss. Thus, a moderate amount of weight loss (7–8%) is sufficient to significantly improve liver histology in patients with NASH. Mehal noted, the “crucial point is that patients do not need to reach their ideal weight, which is very difficult for most patients. We need to ask ourselves what can we really do to help our patients lose weight.” After 6 weeks of treatment with the OPTIFAST VLCD, a mean 43% reduction in liver fat (p=0.016) was evident compared to baseline in subjects with severe obesity prior to undergoing bariatric surgery.45
Mehal concluded that NAFLD and NASH are common, and obesity and metabolic syndrome are key risk factors. Stratification of patients with NAFLD and NASH is a priority and tools to achieve this are not ideal at present. The key predictor of morbidity and mortality is liver fibrosis. Moderate (8–10%) total body weight loss can result in significant improvement in liver histology, and this degree of weight loss can be achieved with a VLCD, such as OPTIFAST. A VLCD along with lifestyle and weight loss medications has an important role in the management of NASH.
Questions and Answers
Did the participants report flavour fatigue with the meal replacement shake?
Flavour fatigue can be experienced with long-term meal replacement use. However, this is something expected as part of the strategy for using meal replacements to narrow the stimuli that individuals are exposed to, thus improving hunger control and reducing cravings.
Should individuals with or without Type 2 diabetes mellitus continue indefinitely on a partial meal replacement diet assuming they are at an appropriate caloric level?
Evidence suggests that using one or two meal replacements a day can be a very useful strategy for long-term maintenance of body weight. There is no evidence to suggest a deleterious effect or concern with long-term use. Some patients use OPTIFAST for 1 month a year as a complementary approach.
Are all meal replacement treatments uniform (i.e., in terms of formulation and calories contained)?
Meal replacement options differ and are formulated based on proprietary differences between available brands. Meal replacement has been described in this symposium in the setting of total diet replacement and is designed and formulated to provide complete nutrition. Over-the-counter products, on the other hand, are not designed to provide full nutrition in the same way and are generally more aptly described as protein supplements. Individuals often use over-the-counter products as snacks rather than replacements, adding to their normal calorie intake, which is not the intent when trying to achieve weight reduction.
Meal replacements are excellent for short-term weight loss through calorie restriction but likely do not change the body weight set point. What about the percentage body weight regain after meal replacement discontinuation and data supporting individual characteristics of responders?
Some treatments are likely more effective than others in modifying energy intake and impacting energy expenditure and possibly lipolysis. However, no available treatment permanently changes the set point. There is some lingering perception that in a total meal replacement diet, rapid weight loss is followed by rapid regain. However, individuals can be supported with longer-term total diet replacement and then be gradually transitioned to food with a partial meal replacement that allows them to continue to lose weight and maintain weight loss longer term. Behavioural counselling in addition to meal replacement is essential to provide subjects with psychological and behavioural tools for weight management. Meal replacement has a very important role as one component within the whole space of weight management.
In terms of the interaction between exercise and liver disease, do any studies show a benefit of exercise independent of weight loss?
Exercise is important, independent of weight loss, and patients should be encouraged to exercise. Liver disease in this context is primarily driven by insulin resistance, and research shows exercise helps build muscle and is associated with better insulin sensitivity. However, precise data to demonstrate this do not exist.
When should a patient transition from a very-low-calorie diet plan?
We restrict total meal replacement to about 12–16 weeks for several reasons. Firstly, to avoid the adaptive response that can lead to a weight loss plateau after this time. To avoid this, the calorie intake should be increased but with a concurrent increase in physical activity to help with energy flux. Thus, more calories are consumed but more calories are also expended allowing for circumvention of the adaptive response. Secondly, to preserve lean mass to help with resting energy expenditure in long-term maintenance of weight loss. Finally, at this time point patients will have a level of self-efficacy and be ready to begin to reintroduce food.
Is there any evidence for use of OPTIFAST 1 month per year for weight maintenance?
At the moment, there is only anecdotal evidence and clinical experience to support use of OPTIFAST for 1 month per year in weight maintenance.
What about people reporting liquids (such as meal replacement drink) not being as filling as solid food?
Satiety is influenced by numerous variables. The content of soluble fibre for example has a significant role to play in satiety and how long patients feel full. Solid foods in general have more fibre than liquid foods, so it would depend on the liquid diet but if patients report feeling less full then this should be accepted. Meal replacements are designed to be complete nutrition and will, despite being in liquid form, give satiety signals to the gut to help signal to the brain that some basic nutritional needs have been met. The brain is not sensitive to the number of calories consumed, but multiple hormones and signals related to carbohydrates, protein, and fat will impact gastric emptying, digestive time, and insulin/glucose levels, which then have an impact on satiety.








